北京大学学报(医学版) ›› 2019, Vol. 51 ›› Issue (3): 591-595. doi: 10.19723/j.issn.1671-167X.2019.03.032
原发性肝癌患者同种异体NK细胞治疗后疗效评价及外周淋巴细胞亚群变化特点
谢云波,张纪元,杜美玲,孟繁平,福军亮,刘利敏,王松山,曲芮,廉方,乔菲,陈杨柳,高莹莹,徐若男,施明,王福生△( )
)
- 解放军总医院第五医学中心感染性疾病诊疗与研究中心, 北京 100039
Efficacy and peripheral immunity analysis of allogeneic natural killer cells therapy in patients with hepatocellular carcinoma
Yun-bo XIE,Ji-yuan ZHANG,Mei-ling DU,Fan-ping MENG,Jun-liang FU,Li-min LIU,Song-shan WANG,Rui QU,Fang LIAN,Fei QIAO,Yang-liu CHEN,Ying-ying GAO,Ruo-nan XU,Ming SHI,Fu-sheng WANG△( )
)
- Institute of Infectious Diseases, the Fifth Medical Center of PLA General Hospital, Beijing 100039, China
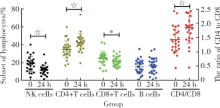
摘要: 目的 评估同种异体自然杀伤(natural killer, NK)细胞治疗原发性肝癌的安全性和有效性,并探讨其抗肿瘤机制。方法 对21例经同种异体NK细胞治疗的原发性肝细胞癌患者进行1年随访,通过对其生存期、影像学指标、血液学指标、生物化学指标及免疫学指标分析,评价该治疗的安全性和有效性,分析患者经过治疗后外周血淋巴细胞亚群的变化。结果 (1)21例原发性肝癌患者中,11例患者治疗1次,5例患者治疗2次,5例患者治疗3次,患者治疗后未见严重不良反应,10例患者有一过性发热,1例患者出现肝区疼痛,1例患者出现头痛,未经治疗均于8 h内自行缓解;(2)在一年随访中,总疾病控制率为76.2%,其中Barcelona分期(Barcelona clinic liver cancer, BCLC)A期患者中疾病控制率为100%, 病情稳定(stable disease, SD)10例;BCLC B期患者中疾病控制率为60%,部分缓解(partial response, PR)1例,SD 2例;BCLC C期患者中疾病控制率为50%,完全缓解(complete response, CR)1例,PR 2例;(3)患者治疗后24 h外周血NK细胞和CD8+T细胞频率较治疗前显著降低,CD4+T细胞频率和CD4/CD8较治疗前显著上升。结论 同种异体NK细胞治疗原发性肝细胞癌有很好的安全性和有效性。
中图分类号:
- R735.7
| [1] |
Boni C, Lampertico P, Talamona L , et al. Natural killer cell phenotype modulation and natural killer/T-cell interplay in nucleos(t)ide analogue-treated hepatitis e antigen-negative patients with chronic hepatitis B[J]. Hepatology, 2015,62(6):1697-1709.
doi: 10.1002/hep.28155 |
| [2] |
Cai L, Zhang Z, Zhou L , et al. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients[J]. Clin Immunol, 2008,129(3):428-437.
doi: 10.1016/j.clim.2008.08.012 |
| [3] |
Easom NJW, Stegmann KA, Swadling L , et al. IL-15 overcomes hepatocellular carcinoma-induced NK cell dysfunction[J]. Front Immunol, 2018,9:1009.
doi: 10.3389/fimmu.2018.01009 |
| [4] | Liu P, Chen L, Zhang H. Natural killer cells in liver disease and hepatocellular carcinoma and the NK cell-based immunotherapy [J/OL]. J Immunol Res, 2018, 2018: 1206737.( 2018-09-04)[2019-03-01].https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6142725/ . |
| [5] |
Parkhurst MR, Riley JP, Dudley ME , et al. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression[J]. Clin Cancer Res, 2011,17(19):6287-6297.
doi: 10.1158/1078-0432.CCR-11-1347 |
| [6] |
Guillerey C, Huntington ND, Smyth MJ . Targeting natural killer cells in cancer immunotherapy[J]. Nat Immunol, 2016,17(9):1025-1036.
doi: 10.1038/ni.3518 |
| [7] |
Eisenhauer EA, Therasse P, Bogaerts J , et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1)[J]. Eur J Cancer, 2009,45(2):228-247.
doi: 10.1016/j.ejca.2008.10.026 |
| [8] |
Wong PPC, Kariminia A, Jones D , et al. Plerixafor effectively mobilizes CD56 (bright) NK cells in blood, providing an allograft predicted to protect against GVHD[J]. Blood, 2018,131(25):2863-2866.
doi: 10.1182/blood-2018-03-836700 |
| [9] | Ullrich E, Salzmann-Manrique E, Bakhtiar S , et al. Relation between acute GVHD and NK cell subset reconstitution following allogeneic stem cell transplantation[J]. Front Immunol, 2016,7:595. |
| [10] |
Kariminia A, Ivison S, Ng B , et al. CD56 (bright) natural killer regulatory cells in filgrastim primed donor blood or marrow products regulate chronic graft-versus-host disease: the Canadian Blood and Marrow Transplant Group randomized 0601 study results[J]. Haematologica, 2017,102(11):1936-1946.
doi: 10.3324/haematol.2017.170928 |
| [11] |
Qin Z, Chen J, Zeng J , et al. Effect of NK cell immunotherapy on immune function in patients with hepatic carcinoma: a preliminary clinical study[J]. Cancer Biol Ther, 2017,18(5):323-330.
doi: 10.1080/15384047.2017.1310346 |
| [12] |
Miller JS, Soignier Y, Panoskaltsis-Mortari A , et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer[J]. Blood, 2005,105(8):3051-3057.
doi: 10.1182/blood-2004-07-2974 |
| [13] |
Llovet JM, Ricci S, Mazzaferro V , et al. Sorafenib in advanced hepatocellular carcinoma[J]. N Engl J Med, 2008,359(4):378-390.
doi: 10.1056/NEJMoa0708857 |
| [14] |
Kohga K, Takehara T, Tatsumi T , et al. Sorafenib inhibits the shedding of major histocompatibility complex class Ⅰ-related chain A on hepatocellular carcinoma cells by down-regulating a disintegrin and metalloproteinase 9[J]. Hepatology, 2010,51(4):1264-1273.
doi: 10.1002/hep.23456 |
| [15] |
Sprinzl MF, Reisinger F, Puschnik A , et al. Sorafenib perpetuates cellular anticancer effector functions by modulating the crosstalk between macrophages and natural killer cells[J]. Hepatology, 2013,57(6):2358-2368.
doi: 10.1002/hep.26328 |
| [1] | 庞泳,张沙,杨华,周柔丽. LAPTM4B-35蛋白作为肝癌血清学诊断新标志物的探讨[J]. 北京大学学报(医学版), 2021, 53(4): 710-715. |
| [2] | 马博,田志华,曲莉,刘月香,张宏,丁慧荣. 索拉菲尼诱导的肝癌耐药细胞株的建立及基因表达分析[J]. 北京大学学报(医学版), 2020, 52(2): 207-213. |
| [3] | 张婧,陈洁,关贵文,张婷,鲁凤民,陈香梅. 肝细胞癌中趋化因子CXCL10及其受体CXCR3的表达及临床意义分析[J]. 北京大学学报(医学版), 2019, 51(3): 402-408. |
|
||