北京大学学报(医学版) ›› 2022, Vol. 54 ›› Issue (5): 1038-1046. doi: 10.19723/j.issn.1671-167X.2022.05.034
血浆置换治疗新月体型IgA肾病的有效性分析: 多中心队列研究
王梓1,张军军2,左力3,王悦4,李文歌5,程虹6,蔡广研7,裴华颖8,王利华9,周绪杰1,师素芳1,刘立军1,吕继成1,*( ),张宏1
),张宏1
- 1. 北京大学第一医院肾内科,北京大学肾脏疾病研究所,卫生部肾脏疾病重点实验室,慢性肾脏病防治教育部重点实验室(北京大学),中国医学科学院免疫介导肾病诊治创新单元,北京 100034
2. 郑州大学附属第一医院肾内科,郑州 450052
3. 北京大学人民医院肾内科,北京 100044
4. 北京大学第三医院肾内科,北京 100191
5. 中日友好医院肾内科,北京 100029
6. 首都医科大学附属北京安贞医院肾内科,北京 10029
7. 中国人民解放军总医院肾内科,北京 100853
8. 河北医科大学第二医院肾内科,石家庄 050000
9. 山西医科大学第二医院肾内科,太原 030001
Efficacy of plasma exchange in severe crescentic IgA nephropathy: A multicentered, cohort study
Zi WANG1,Jun-jun ZHANG2,Li ZUO3,Yue WANG4,Wen-ge LI5,Hong CHENG6,Guang-yan CAI7,Hua-ying PEI8,Li-hua WANG9,Xu-jie ZHOU1,Su-fang SHI1,Li-jun LIU1,Ji-cheng LV1,*( ),Hong ZHANG1
),Hong ZHANG1
- 1. Renal Division, Peking University First Hospital; Institute of Nephrology, Peking University; Key Laboratory of Renal Disease, Ministry of Health of China; Key Laboratory of CKD Prevention and Treatment, Ministry of Education of China, Research Units of Diagnosis and Treatment of lmmune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing 100191, China
2. Department of Nephrology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, 450052, China
3. Department of Nephrology, Peking University People's Hospital, Beijing 100044, China
4. Department of Nephrology, Peking University Third Hospital, Beijing 100191, China
5. Department of Nephrology, China-Japan Friendship Hospital, Beijing 100029, China
6. Division of Nephrology, Beijing Anzhen Hospital, Capital Medical University, Beijing 10029, China
7. Department of Nephrology, Chinese PLA General Hospital, Beijing 100853, China
8. Renal Division, Department of Medicine, The Second Hospital of Hebei Medical University, Shijiazhuang 050000, China
9. Renal Division, Shanxi Medical University Second Affiliated Hospital, Taiyuan 030001, China
摘要:
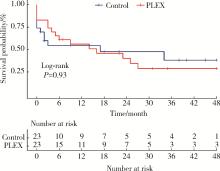
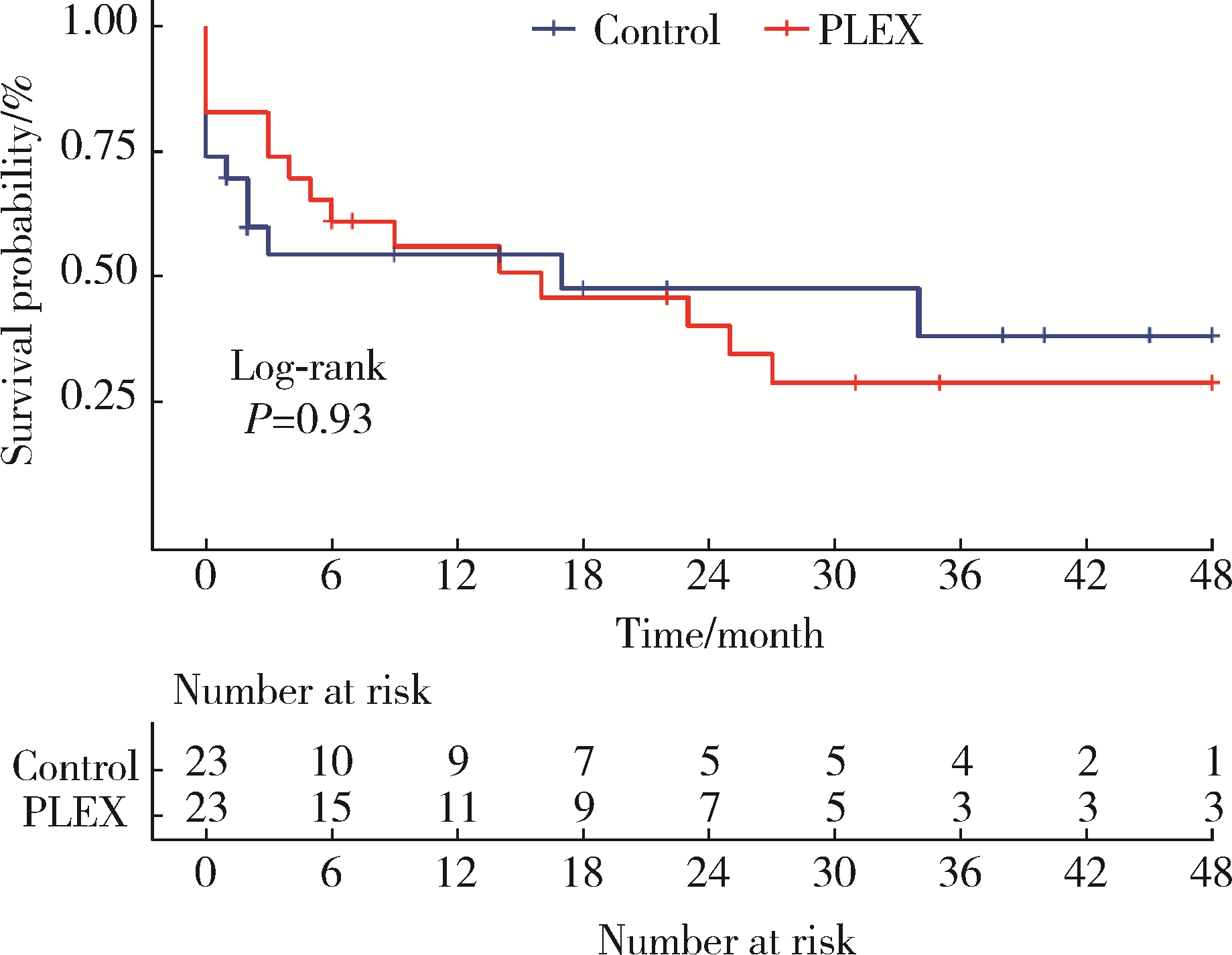
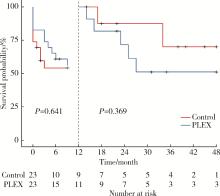
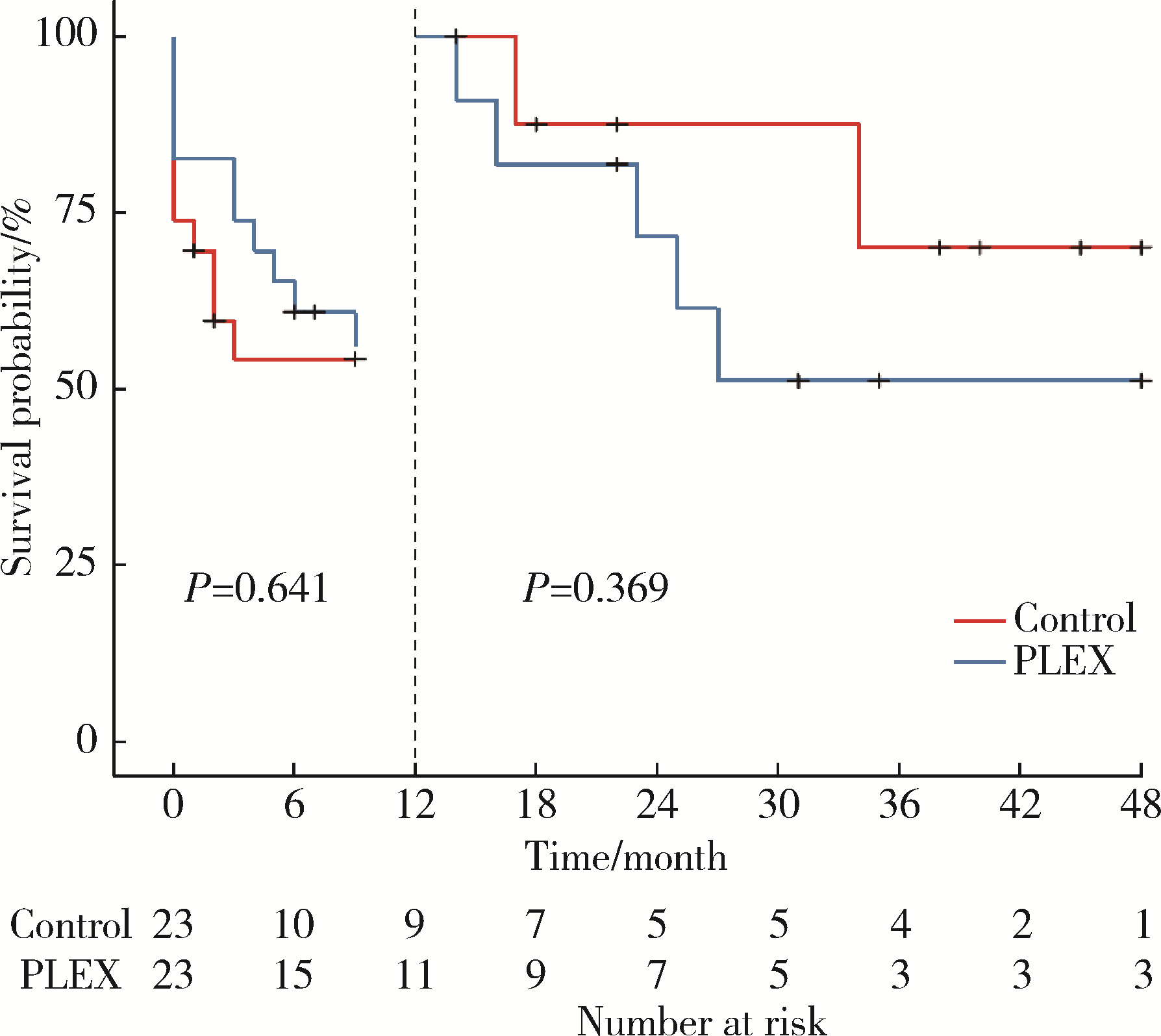
目的: 探究血浆置换治疗新月体型IgA肾病(IgA nephropathy, IgAN)的有效性。方法: 选择2012年1月—2020年9月在北京大学第一医院肾内科等国内9家医院经肾活检确诊为原发性新月体型IgAN的患者病例资料进行回顾性分析,收集患者基线的临床、病理资料及治疗方案信息。为了尽可能减少基线特征中潜在混杂因素的影响,研究采用倾向性评分最近邻匹配法1 ∶1匹配血浆置换组和常规强化免疫抑制治疗组患者临床及病理信息。研究以终末期肾病(end-stage kidney disease, ESKD)为主要结局,采用Kaplan-Meier方法比较两组患者肾生存差异。结果: 共纳入95例新月体型IgA肾病伴急性肾病(acute kidney disease, AKD)患者,其中37例患者接受了血浆置换治疗,58例患者接受常规强化免疫抑制治疗。整体人群肾活检时估算肾小球滤过率(estimated glomerular filtration rate, eGFR)[M(P25, P75)]12.77 (7.28, 21.29) mL/(min·1.73 m2),24-h尿蛋白定量5.9 (4.0, 8.9) g,新月体百分比为64.71%(54.55%, 73.68%)。倾向性评分匹配共23对患者,中位随访时间7 (1, 26) 个月,共29例(63.0%)患者进入终末期肾病, 其中血浆置换治疗组16例(69.6%),常规强化免疫抑制治疗组13例(56.5%)。两组患者基线eGFR[14.30(9.31~17.58) mL/(min·1.73 m2) vs. 11.45(5.59~20.79) mL/(min·1.73 m2)]、24-h尿蛋白定量[(7.4±3.4) g vs. (6.6±3.8) g]、新月体百分比(64.49%±13.23% vs. 66.41%±12.65%)、肾活检后应用激素治疗比例[23(100.0%) vs. 21(91.3%)] 比较,差异均无统计学意义(P均>0.05)。Kaplan-Meier生存分析结果示生存率组间比较,差异无统计学意义(Log-rank检验,P=0.933)。结论: 在常规强化免疫抑制治疗的基础上加用血浆置换治疗未能进一步改善新月体型IgA肾病预后。
中图分类号:
- R692
| 1 |
Cattran DC , Feehally J , Cook HT , et al. Kidney disease: Improving global outcomes (KDIGO) glomerulonephritis work group. KDIGO clinical practice guideline for glomerulonephritis[J]. Kidney Int, 2012, 2 (2): 139- 274.
doi: 10.1038/kisup.2012.9 |
| 2 | Kidney disease: Improving global outcomes (KDIGO) glomerular diseases work group . KDIGO 2021 Clinical practice guideline for the management of glomerular diseases[J]. Kidney Int, 2021, 100 (Suppl 4): S1- S276. |
| 3 |
Lv J , Yang Y , Zhang H , et al. Prediction of outcomes in crescentic IgA nephropathy in a multicenter cohort study[J]. J Am Soc Nephrol, 2013, 24 (12): 2118- 2125.
doi: 10.1681/ASN.2012101017 |
| 4 |
Le vy , Jeremy B . Long-term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression[J]. Ann Intern Med, 2001, 134 (11): 1033- 1042.
doi: 10.7326/0003-4819-134-11-200106050-00009 |
| 5 |
Jayne DR , Gaskin G , Rasmussen N , et al. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis[J]. J Am Soc Nephrol, 2007, 18 (7): 2180- 2188.
doi: 10.1681/ASN.2007010090 |
| 6 |
Suzuki H , Kiryluk K , Novak J , et al. The Pathophysiology of IgA nephropathy[J]. J Am Soc Nephrol, 2011, 22 (10): 1795- 1803.
doi: 10.1681/ASN.2011050464 |
| 7 |
Chambers ME , McDonald BR , Hall FW , et al. Plasmapheresis for crescentic IgA nephropathy: A report of two cases and review of the literature[J]. J Clin Apher, 1999, 14 (4): 185- 187.
doi: 10.1002/(SICI)1098-1101(1999)14:4<185::AID-JCA6>3.0.CO;2-K |
| 8 | Roccatello D , Ferro M , Coppo R , et al. Report on intensive treatment of extracapillary glomerulonephritis with focus on cresentic IgA nephropathy[J]. Nephrol Dial Transplant, 1995, 10 (11): 2054- 2059. |
| 9 |
Fujinaga S , Ohtomo Y , Umino D , et al. Plasma exchange combined with immunosuppressive treatment in a child with rapidly progressive IgA nephropathy[J]. Pediatr Nephrol, 2007, 22 (6): 899- 902.
doi: 10.1007/s00467-006-0428-4 |
| 10 |
Xie X , Lv J , Shi S , et al. Plasma exchange as an adjunctive therapy for crescentic IgA nephropathy[J]. Am J Nephrol, 2016, 44 (2): 141- 149.
doi: 10.1159/000448767 |
| 11 |
Padmanabhan A , Connelly-Smith L , Aqui N , et al. Guidelines on the use of therapeutic apheresis in clinical practice-evidence based approach from the writing committee of the american society for apheresis: The eighth special issue[J]. J Clin Apher, 2019, 34 (3): 171- 354.
doi: 10.1002/jca.21705 |
| 12 |
Wyatt RJ , Julian BA . IgA nephropathy[J]. N Engl J Med, 2013, 368 (25): 2402- 2414.
doi: 10.1056/NEJMra1206793 |
| 13 |
Yu G , Zhang Y , Meng B , et al. O-glycoforms of polymeric immunoglobulin A1 in the plasma of patients with IgA nephropathy are associated with pathological phenotypes[J]. Nephrol Dial Transplant, 2021, 37 (1): 33- 41.
doi: 10.1093/ndt/gfab204 |
| 14 | Yamazaki Y , Mori A , Nomura Y , et al. A case of rapidly progressive IgA nephropathy with transient hypocomplementemia at onset[J]. Nihon Jinzo Gakkai Shi, 1997, 39 (7): 765- 770. |
| 15 | Coppo R , Basolo B , Roccatello D , et al. Immunological monitoring of plasma exchange in primary IgA nephropathy[J]. Artif Organs, 2010, 9 (4): 351- 360. |
| 16 |
Lai KN , Lai FM , Leung AC , et al. Plasma exchange in patients with rapidly progressive idiopathic IgA nephropathy: A report of two cases and review of literature[J]. Am J Kidney Dis, 1987, 10 (1): 66- 70.
doi: 10.1016/S0272-6386(87)80014-8 |
| 17 | Wang Z, Jiang Y, Chen P, et al. The level of urinary C4d is associated with disease progression in IgA nephropathy with glomerular crescentic lesions: A cohort study[J]. Nephrol Dial Transplant, 2022[2022-06-01]. https://academic.oup.com/ndt/advance-article-abstract/doi/10.1093/ndt/gfac024/6519273?redirectedFrom=fulltext&login=false |
| 18 |
Rosenblad T , Rebetz J , Johansson M , et al. Eculizumab treatment for rescue of renal function in IgA nephropathy[J]. Pediatr Nephrol, 2014, 29 (11): 2225- 2228.
doi: 10.1007/s00467-014-2863-y |
| 19 |
Troels R , Bang P B , Giedrius S , et al. Use of eculizumab in crescentic IgA nephropathy: Proof of principle and conundrum[J]. Clin Kidney J, 2015, 8 (5): 489- 491.
doi: 10.1093/ckj/sfv076 |
| 20 | Matsumura D , Tanaka A , Nakamura T , et al. Coexistence of atypical hemolytic uremic syndrome and crescentic IgA nephropathy treated with eculizumab: A case report[J]. Clin Nephrol Case Stud, 2016, 4, 24- 28. |
| 21 |
Lafayette RA , Rovin BH , Reich HN , et al. Safety, tolerability and efficacy of narsoplimab, a novel MASP-2 inhibitor for the treatment of IgA nephropathy[J]. Kidney Int Rep, 2020, 5 (11): 2032- 2041.
doi: 10.1016/j.ekir.2020.08.003 |
| [1] | 包文晗,唐雯. 初诊IgA肾病患者的肠道菌群及其与疾病进展因素的相关分析[J]. 北京大学学报(医学版), 2023, 55(1): 124-132. |
| [2] | 贾金凤,梁菲,黄建伟,王昊,韩璞青. 双重血浆分子吸附系统模式人工肝治疗对血小板的影响[J]. 北京大学学报(医学版), 2022, 54(3): 548-551. |
| [3] | 石茂静,高伟波,黄文凤,朱继红. 61例血栓性血小板减少性紫癜患者的临床分析[J]. 北京大学学报(医学版), 2021, 53(1): 210-214. |
| [4] | 康玉琦,张月苗,侯平,师素芳,刘立军,周绪杰,吕继成,张宏. IgA肾病易感基因遗传多态性的种族差异分析[J]. 北京大学学报(医学版), 2019, 51(3): 459-466. |
| [5] | 伍刚,彭佑铭,徐道亮,刘昌华. 腭扁桃体及外周血中记忆B细胞在IgA肾病临床进展中的异常表达[J]. 北京大学学报(医学版), 2015, 47(5): 749-753. |
| [6] | 肖慧捷, 何瑞娟. 补体系统调控异常与C3肾小球病[J]. 北京大学学报(医学版), 2013, 45(2): 323-. |
| [7] | 刘颖, 陈育青, 周晶晶, 韩佳, 梁彧, 李雪迎, 张宏. 尿调蛋白酶联免疫吸附试验检测方法的建立及其在IgA肾病中的应用[J]. 北京大学学报(医学版), 2012, 44(2): 307-310. |
| [8] | 邹古明, 谌贻璞, 李文歌. 水痘病毒感染伴肾小球肾炎和脑炎1例报告(英文稿)[J]. 北京大学学报(医学版), 2011, 43(6): 914-918. |
| [9] | 李珺, 曲贞, 张宜苗, 于峰, 黄婧, 杨瑞, 赵明辉, 刘刚. 检测血、尿IgG4在特发性膜性肾病中的临床意义[J]. 北京大学学报(医学版), 2010, 42(6): 671-674. |
| [10] | 杨艳荣, 吕继成, 蒋镭, 张宜苗, 宋玉红, 李荣山, 张宏. 老年IgA肾病的临床病理特征和预后分析[J]. 北京大学学报(医学版), 2008, 40(4): 401-404. |
| [11] | 冯现竹, 侯平, 朱厉, 于磊, 张宏. 转铁蛋白受体基因多态性与IgA肾病易感性及临床病理表型的相关性[J]. 北京大学学报(医学版), 2008, 40(4): 369-373. |
| [12] | 李月婷, 吕继成, 李光韬, 蒋镭, 宋玉红, 张宏. 成人过敏性紫癜性肾炎与IgA肾病临床病理和转归对比分析[J]. 北京大学学报(医学版), 2007, 39(5): 458-461. |
| [13] | 李惊子, 黄海长, 刘颖, 鄂杰. 检测尿足细胞在活动性肾小球疾病中的意义[J]. 北京大学学报(医学版), 2005, 37(5): 463-466. |
| [14] | 张军军, 于峰, 徐丽霞, 张颖, 赵明辉. 糖基化缺陷的血清IgA1与人脐静脉内皮细胞结合量的研究[J]. 北京大学学报(医学版), 2005, 37(2): 139-142. |
| [15] | 熊祖应, 黄海长, 李惊子, 王海燕. 活动性肾小球肾炎中过氧化脂质体增殖激活受体γ在肾脏的表达[J]. 北京大学学报(医学版), 2003, 35(5): 499-502. |
|
||