北京大学学报(医学版) ›› 2025, Vol. 57 ›› Issue (2): 237-244. doi: 10.19723/j.issn.1671-167X.2025.02.003
miR-34a在高氧诱导新生大鼠支气管肺发育不良模型中的表达及调控机制
- 内蒙古医科大学附属医院新生儿科,呼和浩特 010050
Expression and regulatory mechanism of miR-34a in neonatal rat model of bron-chopulmonary dysplasia induced by hyperoxia
Mengyue HUO, Hua MEI*( ), Yuheng ZHANG, Yanbo ZHANG, Chunli LIU
), Yuheng ZHANG, Yanbo ZHANG, Chunli LIU
- Department of Neonatology, Affiliated Hospital of Inner Mongolia Medical University, Hohhot 010050, China
摘要:
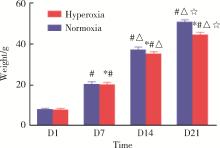
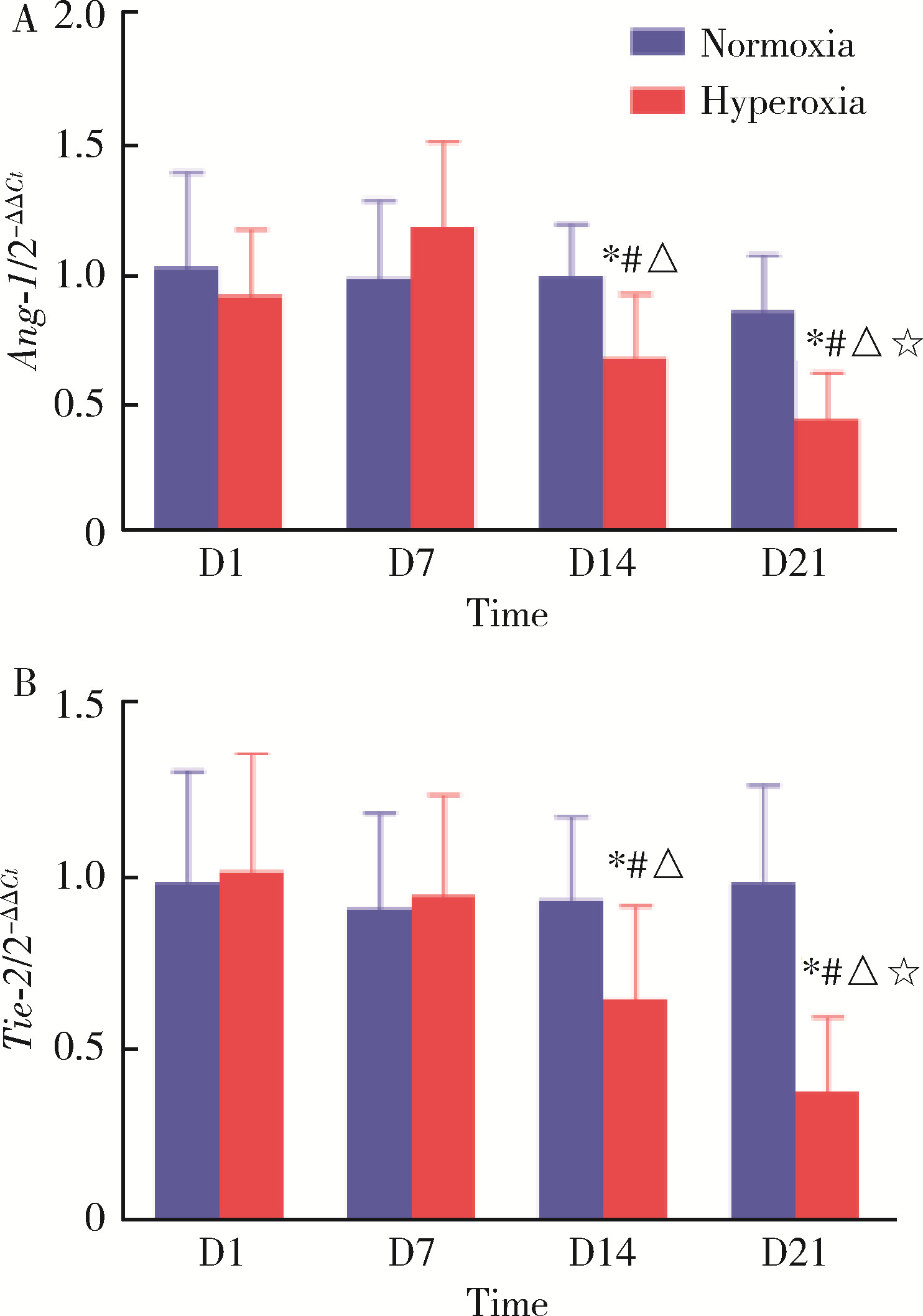
目的: 探讨miR-34a在高氧诱导新生大鼠支气管肺发育不良(bronchopulmonary dysplasia,BPD)模型肺组织中的表达及其可能的调控机制。方法: 将80只新生SD大鼠于生后2 h内随机分配到高氧组(FiO2=60%)及空气组(FiO2=21%),每组40只,分别于生后第1、7、14、21天提取各组SD大鼠肺组织标本,HE染色后于光镜下观察肺组织病理变化,记录辐射状肺泡计数(radial alveolar counts,RAC),并测量平均肺泡直径(mean alveolar dia-meter,MAD)和肺泡间隔厚度(alveolar septal thickness,AST)以评价肺泡发育情况;应用实时荧光定量PCR技术检测不同时间点高氧组与空气组大鼠肺组织中miR-34a、血管生成素-1(angiopoietin-1,Ang-1)和酪氨酸激酶受体-2(tyrosine kinase receptor-2,Tie-2)的基因表达情况;应用酶联免疫吸附测定法(enzyme linked immunosorbent assays,ELISA)检测不同时间点两组大鼠肺组织中Ang-1及Tie-2蛋白的表达水平。结果: 高氧组大鼠生后第7、14、21天体重较空气组降低,差异有统计学意义(P均 < 0.05)。高氧组大鼠肺组织随氧暴露时间的延长逐渐出现肺泡数量减少、体积增大、结构简单化、肺泡腔明显增大和肺泡间隔增厚等肺发育受阻表现;高氧组大鼠生后第7、14、21天RAC较空气组明显减少,差异有统计学意义(P均 < 0.05)。与空气组相比,高氧组大鼠生后第7、14、21天MAD和AST明显增加,差异有统计学意义(P均 < 0.05)。高氧组大鼠肺组织miR-34a在生后第7、14、21天的表达水平均明显高于空气组,差异有统计学意义(P均 < 0.05)。与同时间点空气组相比,高氧组大鼠肺组织中Ang-1和Tie-2 mRNA的表达水平和蛋白质的表达水平在生后第14、21天均低于空气组,差异有统计学意义(P均 < 0.05)。结论: 将新生SD大鼠持续暴露于60%的高氧环境中可成功构建大鼠新型BPD模型,在新生大鼠新型BPD模型的肺组织中miR-34a表达上调,miR-34a可能通过调控Ang-1/Tie-2信号通路在BPD的发生发展中起到重要作用。
中图分类号:
- R722.1
| 1 |
Jensen EA , Edwards EM , Greenberg LT , et al. severity of bronchopulmonary dysplasia among very preterm infants in the United States[J]. Pediatrics, 2021, 148 (1): e2020030007.
doi: 10.1542/peds.2020-030007 |
| 2 |
Ronkainen E , Perhomaa M , Mattila L , et al. Structural pulmonary abnormalities still evident in schoolchildren with new bronchopulmonary dysplasia[J]. Neonatology, 2018, 113 (2): 122- 130.
doi: 10.1159/000481356 |
| 3 |
Dang MN , Gomez Casas C , Day ES , et al. Photoresponsive miR-34a/nanoshell conjugates enable light-triggered gene regulation to impair the function of triple-negative breast cancer cells[J]. Nano Lett, 2021, 21 (1): 68- 76.
doi: 10.1021/acs.nanolett.0c03152 |
| 4 |
Bhaskaran M , Xi D , Wang Y , et al. Idenfification of microRNAs changed in the neonatal lungs in response to hyperoxia exposure[J]. Physiol Genomics, 2012, 44 (20): 970- 980.
doi: 10.1152/physiolgenomics.00145.2011 |
| 5 |
Kim DH , Kim HS . Serial changes of serum endostatin and angiopoietin-1 levels in preterm infants with severe bronchopulmonary dysplasia and subsequent pulmonary arteryhypertension[J]. Neonatolog, 2014, 106 (1): 55- 61.
doi: 10.1159/000358374 |
| 6 |
Dumpa V , Nielsen L , Wang H , et al. Caffeine is associated with improved alveolarization and angiogenesis in malemice following hyperoxia induced lung injury[J]. BMC Pulm Med, 2019, 19 (1): 138.
doi: 10.1186/s12890-019-0903-x |
| 7 |
Syed M , Das P , Pawar A , et al. Hyperoxia causes miR-34a-mediated injury via angiopoietin-1 in neonatal lungs[J]. Nat Commun, 2017, 8 (1): 1173.
doi: 10.1038/s41467-017-01349-y |
| 8 | 冯洁, 邓春, 余加林, 等. 高迁移率族蛋白B1在高氧致支气管肺发育不良的表达[J]. 中国当代儿科杂志, 2010, 12 (3): 219- 223. |
| 9 |
Braun RK , Chetty C , Balasubramaniam V , et al. Intraperitoneal injection of MSC-derived exosomes prevent experimental bronc-hopulmonary dysplasia[J]. Biochem Biophys Res Commun, 2018, 503 (4): 2653- 2658.
doi: 10.1016/j.bbrc.2018.08.019 |
| 10 | Mei Y , Chen C , Dong H , et al. treatment of hyperoxia-induced lung injury with lung mesenchymal stem cells in mice[J]. Stem Cells Int, 2018, 2018, 5976519. |
| 11 |
Porzionato A , Guidolin D , Macchi V , et al. Fractal analysis of alveolarization in hyperoxia-induced rat models of bronchopulmonary dysplasia[J]. Am J Physiol Lung Cell Mol Physiol, 2016, 310 (7): L680- L688.
doi: 10.1152/ajplung.00231.2015 |
| 12 |
Li J , Yu KH , Oehlert J , et al. Exome sequencing of neonatal blood spots and the identification of genes implicated in bron-chopulmonary dysplasia[J]. Am J Respir Crit Care Med, 2015, 192 (5): 589- 596.
doi: 10.1164/rccm.201501-0168OC |
| 13 | Alam MA , Betal SGN , Aghai ZH , et al. Hyperoxia causes miR199a-5p-mediated injury in the developing lung[J]. Pediatr Res, 2019, 85 (5): 579- 588. |
| 14 |
张潇月, 蔡成, 楚晓云, 等. 高体积分数氧暴露对早产新生大鼠肺组织微小RNA-125b、肿瘤坏死因子-α和白细胞介素6表达的影响[J]. 中华实用儿科临床杂志, 2019, 34 (16): 1244- 1248.
doi: 10.3760/cma.j.issn.2095-428X.2019.16.013 |
| 15 | 月小飞, 梅花, 宋丹, 等. 高氧诱导支气管肺发育不良模型新生大鼠肺组织中miR-21-5p的表达[J]. 中国医科大学学报, 2020, 49 (7): 624-627, 635. |
| 16 | 孙祎璠, 马俐, 龚小慧, 等. 基于生物信息学分析microRNA-495-5p在早产儿支气管肺发育不良中的表达及其临床意义[J]. 中国当代儿科杂志, 2020, 22 (1): 24- 30. |
| 17 |
van den Berge M , Tasena H . Role of microRNAs and exosomes in asthma[J]. Curr Opin Pulm Med, 2019, 25 (1): 87- 93.
doi: 10.1097/MCP.0000000000000532 |
| 18 |
Das P , Syed MA , Shah D , et al. miR34a: A master regulator in the pathogenesis of bronchopulmonary dysplasia[J]. Cell Stress, 2018, 2 (2): 34- 36.
doi: 10.15698/cst2018.02.124 |
| 19 |
Ruiz-Camp J , Quantius J , Lignelli E , et al. Targeting miR-34a/Pdgfra interactions partially corrects alveologenesis in experimental bronchopulmonary dysplasia[J]. EMBO Mol Med, 2019, 11 (3): e9448.
doi: 10.15252/emmm.201809448 |
| 20 |
Liu H , Li S , Xu Y , et al. Engeletin protects against cerebral ischemia/reperfusion injury by modulating the VEGF/vasohibin and Ang-1/Tie-2 pathways[J]. Braz J Med Biol Res, 2021, 54 (10): e11028.
doi: 10.1590/1414-431x2020e11028 |
| 21 |
王玲, 吕回, 李美雪. 血管生成素-1在高氧诱导新生鼠支气管肺发育不良的表达及与肺发育的关系[J]. 临床儿科杂志, 2014 (4): 355- 359.
doi: 10.3969/j.issn.1000-3606.2014.04.017 |
| 22 |
Sudhadevi T , Jafri A , Ha AW , et al. Hyperoxia-induced S1P signaling reduced angiogenesis by suppression of Tie-2 leading to experimental bronchopulmonary dysplasia[J]. Cell Biochem Biophys, 2021, 79 (3): 561- 573.
doi: 10.1007/s12013-021-01014-8 |
| [1] | 包菊,刘佳,曲元,穆东亮. 脐动脉血气pH值对剖宫产新生儿住院期间并发症的预测价值[J]. 北京大学学报(医学版), 2019, 51(1): 159-164. |
| [2] | 张欣,茹喜芳,王颖,李星,桑田,冯琪. 新生儿重症监护病房中新生儿真菌败血症的临床特点[J]. 北京大学学报(医学版), 0, (): 789-793. |
| [3] | 张欣, 茹喜芳, 王颖, 李星, 桑田, 冯琪. 新生儿重症监护病房中新生儿真菌败血症的临床特点[J]. 北京大学学报(医学版), 2017, 49(5): 789-793. |
|
||