北京大学学报(医学版) ›› 2025, Vol. 57 ›› Issue (2): 253-261. doi: 10.19723/j.issn.1671-167X.2025.02.005
长效重组人白介素2药物的生物学活性和抑瘤作用
- 浙江新码生物医药有限公司,浙江绍兴 312000
Biological activity and antitumor effect of long-acting recombinant human interleukin-2 drug
Xuejun LIANG, Fengxia ZHANG, Ting JIN, Jingjing ZHU*( )
)
- Novocodex Biopharmaceuticals Company Limited, Shaoxing 312000, Zhejiang, China
摘要:
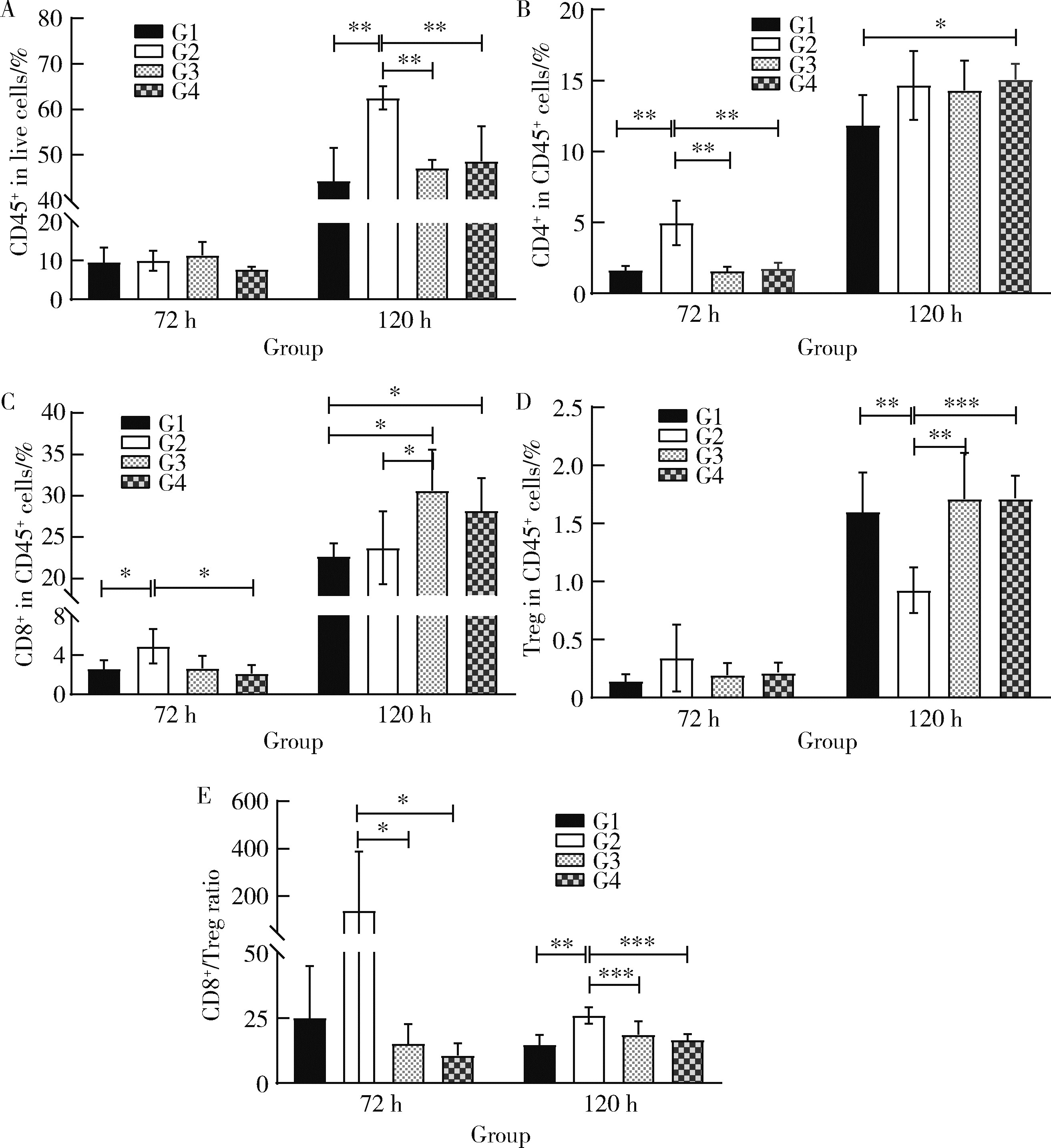
目的: 考察运用非天然氨基酸定点偶联聚乙二醇(polyethylene glycol,PEG)获得的PEG化重组人白介素2(pegylated recombinant human interleukin 2,PEG-rhIL-2)的生物学活性和抑瘤作用,并探索其抑瘤机制。方法: 用表面等离子共振(surface plasmon resonance,SPR)技术检测T41、Y45和V91三个不同位点PEG-rhIL-2与人IL-2受体α(interleukin 2 receptor α,IL-2Rα)和IL-2受体β(interleukin 2 receptor β,IL-2Rβ)的平衡解离常数(equilibrium dissociation constant,KD)。采用Western blot检测不同剂量rhIL-2和PEG-rhIL-2激活CTTL-2和YT细胞中Janus激酶-信号转导和转录激活因子5(Janus kinase-signal transducer and activator of transcription 5,JAK-STAT5)信号通路的水平。将小鼠单次给药后采血,检测不同时间点药物的浓度,评估Y45-PEG-rhIL-2的药代动力学参数。选择小鼠肝癌细胞系Hepa1-6、胰腺癌细胞系Pan-02和结肠癌细胞系MC-38,用C57BL/6品系小鼠构建肿瘤模型,分别注射不同剂量的Y45-PEG-rhIL-2及赋形剂对照,评估药物的抑瘤作用,并在MC-38模型中评估Y45-PEG-rhIL-2与抗小鼠程序性死亡受体-1(programmed death-1,PD-1)单抗联用的抑瘤作用。构建Hepa1-6小鼠肿瘤模型,分别注射rhIL-2、Y45-rhIL-2和Y45-PEG-rhIL-2,用流式细胞仪分析肿瘤浸润淋巴细胞的比例。结果: SPR检测结果显示PEG-rhIL-2与IL-2Rα/IL-2Rβ亲和力均降低,Y45-PEG-rhIL-2与IL-2Rα亲和力降低至约1/250,与IL-2Rβ亲和力降低至1/3。Western blot结果显示Y45-PEG-rhIL-2激活表达IL-2受体异源三聚体αβγ(heterotrimeric IL-2 receptor complex αβγ,IL-2Rαβγ)的CTLL-2细胞JAK-STAT5信号的活性降低至约1/300,激活表达IL-2受体异源二聚体βγ(heterodimeric IL-2 receptor complex βγ,IL-2Rβγ)的YT细胞JAK-STAT5信号的活性降低至1/3。小鼠单次给药后药代动力学评估结果显示Y45-PEG-rhIL-2消除半衰期为17.7 h,具有优于rhIL-2的药代动力学特征。在小鼠肿瘤模型中,Y45-PEG-rhIL-2显示出剂量依赖的抑瘤作用,且与抗PD-1抗体联用抑瘤作用优于Y45-PEG-rhIL-2单用和抗PD-1抗体单用。流式结果表明Y45-PEG-rhIL-2给药72 h后,肿瘤浸润CD8+T细胞比例增加86.84%,给药120 h后CD8+T细胞与调节T细胞(regulatory T cell,Treg)比值提高75.10%。结论: 采用非天然氨基酸定点偶联得到的Y45-PEG-rhIL-2通过改变受体亲和力,调节CD8+T细胞杀伤肿瘤,在小鼠多个移植瘤模型上具有剂量依赖的抑瘤作用。
中图分类号:
- R966
| 1 | Bray F , Ferlay J , Soerjomataram I , et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68 (6): 394- 424. |
| 2 |
Bray F , Laversanne M , Sung H , et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2024, 74 (3): 229- 263.
doi: 10.3322/caac.21834 |
| 3 |
Sung H , Ferlay J , Siege RL , et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71 (3): 209- 249.
doi: 10.3322/caac.21660 |
| 4 |
Raeber ME , Sahin D , Karakus U , et al. A systematic review of interleukin-2-based immunotherapies in clinical trials for cancer and autoimmune diseases[J]. EBioMedicine, 2023, 90, 104539.
doi: 10.1016/j.ebiom.2023.104539 |
| 5 |
Ritacco C , Ehx G , Gregoire C , et al. High proportion of terminally differentiated regulatory T cells after allogeneic hematopoietic stem cell transplantation[J]. Bone Marrow Transplant, 2021, 56 (8): 1828- 1841.
doi: 10.1038/s41409-021-01221-0 |
| 6 |
Taniguchi T , Minami Y . The IL-2/IL-2 receptor system: A current overview[J]. Cell, 1993, 73 (1): 5- 8.
doi: 10.1016/0092-8674(93)90152-G |
| 7 |
Wang X , Lupardus P , Laporte SL , et al. Structural biology of shared cytokine receptors[J]. Annu Rev Immunol, 2009, 27, 29- 60.
doi: 10.1146/annurev.immunol.24.021605.090616 |
| 8 |
Spolski , Li P , Leonard WJ . Biology and regulation of IL-2: From molecular mechanisms to human therapy[J]. Nat Rev Immunol, 2018, 18 (10): 648- 659.
doi: 10.1038/s41577-018-0046-y |
| 9 |
Boyman O , Sprent J . The role of interleukin-2 during homeostasis and activation of the immune system[J]. Nat Rev Immunol, 2012, 12 (3): 180- 190.
doi: 10.1038/nri3156 |
| 10 |
Fyfe GA , Fisher RI , Rosenberg SA , et al. Long-term response data for 255 patients with metastatic renal cell carcinoma treated with high-dose recombinant interleukin-2 therapy[J]. J Clin Oncol, 1996, 14 (8): 2410- 2411.
doi: 10.1200/JCO.1996.14.8.2410 |
| 11 |
Rosenberg SA , Yang JC , Topalian SL , et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2[J]. JAMA, 1994, 271 (12): 907- 913.
doi: 10.1001/jama.1994.03510360033032 |
| 12 |
Javia L R , Rosenberg SA . CD4+CD25+ suppressor lymphocytes in the circulation of patients immunized against melanoma antigens[J]. J Immunother, 2003, 26 (1): 85- 93.
doi: 10.1097/00002371-200301000-00009 |
| 13 |
Yang JC , Sherry RM , Steinberg SM , et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer[J]. J Clin Oncol, 2003, 21 (16): 3127- 3132.
doi: 10.1200/JCO.2003.02.122 |
| 14 |
Rojas G , Relova-Hernandez E , Perez-Riveron A , et al. Molecular reshaping of phage-displayed Interleukin-2 at beta chain receptor interface to obtain potent super-agonists with improved developability profiles[J]. Commun Biol, 2023, 6 (1): 828.
doi: 10.1038/s42003-023-05188-0 |
| 15 |
Leonard EK , Tomala J , Gould JR , et al. Engineered cytokine/antibody fusion proteins improve IL-2 delivery to pro-inflammatory cells and promote antitumor activity[J]. JCI Insight, 2024, 9 (18): e173469.
doi: 10.1172/jci.insight.173469 |
| 16 |
Pasut G , Veronese FM . State of the art in PEGylation: the great versatility achieved after forty years of research[J]. J Control Release, 2012, 161 (2): 461- 472.
doi: 10.1016/j.jconrel.2011.10.037 |
| 17 |
Shen BQ , Xu K , Liu L , et al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates[J]. Nat Biotechnol, 2012, 30 (2): 184- 189.
doi: 10.1038/nbt.2108 |
| 18 |
Wang L , Brock A , Herberich B , et al. Expanding the genetic code of Escherichia coli[J]. Science, 2001, 292 (5516): 498- 500.
doi: 10.1126/science.1060077 |
| 19 |
Nguyen TTK , Pham KY , Yook S . Engineered therapeutic proteins for sustained-release drug delivery systems[J]. Acta Biomater, 2023, 171, 131- 154.
doi: 10.1016/j.actbio.2023.09.018 |
| 20 |
Charych DH , Hoch U , Langowski JL , et al. NKTR-214, an engineered cytokine with biased IL2 receptor binding, increased tumor exposure, and marked efficacy in mouse tumor models[J]. Clin Cancer Res, 2016, 22 (3): 680- 690.
doi: 10.1158/1078-0432.CCR-15-1631 |
| 21 |
Nagaraja-Shastri P , Zhu J , Skidmore L , et al. Nonclinical deve-lopment of next-generation site-specific HER2-targeting antibody-drug conjugate (ARX788) for breast cancer treatment[J]. Mol Cancer Ther, 2020, 19 (9): 1822- 1832.
doi: 10.1158/1535-7163.MCT-19-0692 |
| 22 |
Zhang B , Sun J , Wang Y , et al. Site-specific PEGylation of interleukin-2 enhances immunosuppression via the sustained activation of regulatory T cells[J]. Nat Biomed Eng, 2021, 5 (11): 1288- 1305.
doi: 10.1038/s41551-021-00797-8 |
| 23 | Sledzinska A , de Mucha MV , Bergerhoff K , et al. Regulatory T cells restrain interleukin-2- and Blimp-1-dependent acquisition of cytotoxic function by CD4+ T Cells[J]. Immunity, 2020, 52 (1): 151. e6- 166. e6. |
| 24 |
Schmidt D , Endres C , Hoefflin R , et al. Oncogenic calreticulin induces iImmune escape by stimulating TGFbeta expression and regulatory T-cell expansion in the bone marrow microenvironment[J]. Cancer Res, 2024, 84 (18): 2985- 3003.
doi: 10.1158/0008-5472.CAN-23-3553 |
| 25 | Xie Q , Tang Z , Liang X , et al. An immune-related gene prognostic index for acute myeloid leukemia associated with regulatory T cells infiltration[J]. Hematology, 2022, 27 (1): 1088- 1100. |
| 26 |
Sharma M , Khong H , Fa'ak F , et al. Bempegaldesleukin selectively depletes intratumoral Tregs and potentiates T cell-mediated cancer therapy[J]. Nat Commun, 2020, 11 (1): 661.
doi: 10.1038/s41467-020-14471-1 |
| 27 | Cheng W , Kang K , Zhao A , et al. Dual blockade immunotherapy targeting PD-1/PD-L1 and CTLA-4 in lung cancer[J]. J Hematol Oncol, 2024, 17 (1): 54. |
| 28 | Liu R , Zeng LW , Li HF , et al. PD-1 signaling negatively regulates the common cytokine receptor gamma chain via MARCH5-mediated ubiquitination and degradation to suppress anti-tumor immunity[J]. Cell Res, 2023, 33 (12): 923- 939. |
| [1] | 刘家骏, 刘国康, 朱玉虎. 免疫相关性重症肺炎1例[J]. 北京大学学报(医学版), 2024, 56(5): 932-937. |
| [2] | 姜妮,乔国梁,王小利,周心娜,周蕾,宋雨光,赵艳杰,任军. 中性粒细胞与淋巴细胞比例对评估接受过继性细胞免疫治疗的晚期胰腺癌患者预后的临床意义[J]. 北京大学学报(医学版), 2020, 52(3): 597-602. |
| [3] | 梁学军,宫丽颖,周菲,周德敏,祝静静. 非天然氨基酸定点偶联抗人类表皮生长因子受体2-抗体偶联药物的药理学活性[J]. 北京大学学报(医学版), 2019, 51(5): 797-804. |
| [4] | 刘静维, 卢戌, 杨照敏, 邓丽娟, 杨林. 负载NY-ESO-1多肽的树突状细胞激发特异性细胞毒性T淋巴细胞反应[J]. 北京大学学报(医学版), 2017, 49(5): 840-846. |
|
||