Journal of Peking University (Health Sciences) ›› 2020, Vol. 52 ›› Issue (3): 570-577. doi: 10.19723/j.issn.1671-167X.2020.03.026
Previous Articles Next Articles
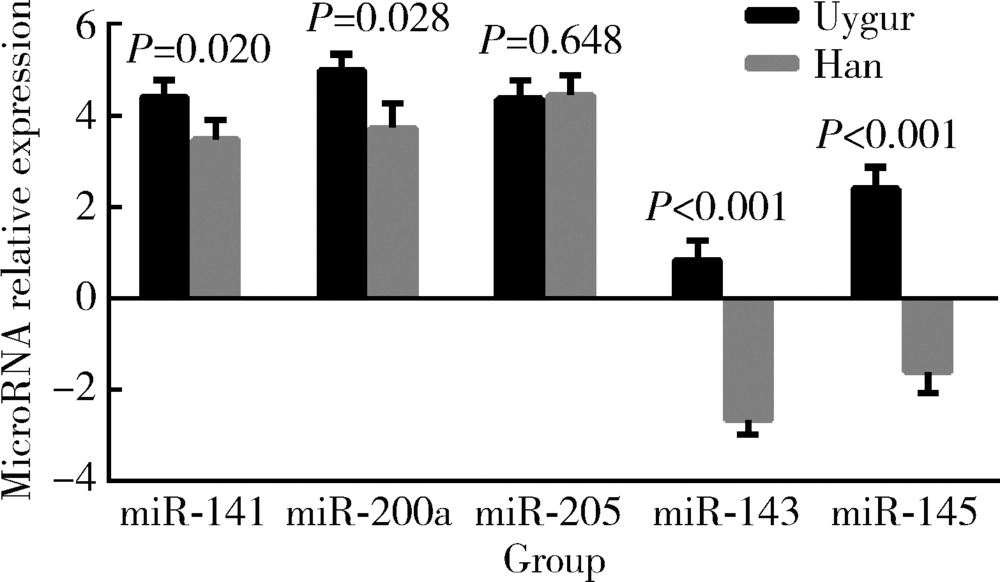
Characteristic and clinical significance of microRNA expression between 144 Uygur and Han women with endometrial carcinoma
Xiao WANG1,Dan HE1,Wen-ting LI2,SIYITI Adila·2,Rui HAN1,Ying DONG1,△( )
)
- 1. Department of Pathology, Peking University First Hospital, Beijing 100034, China
2. Department of Pathology, Affiliated Cancer Hospital of Xinjiang Medical University, Urumqi 830011, China
CLC Number:
- R737.33
| [1] | 廖秦平, 杨曦. 子宫内膜癌筛查及早期诊断的现状及展望. 实用妇产科杂志, 2015,31(7):481-484. |
| [2] | Cancer Genome Atlas Research, Network. Integrated genomic characterization of endometrial carcinoma[J]. Nature, 2013,497(7447):67-73. |
| [3] | Schimp VL, Ali-Fehmi R, Solomon LA, et al. The racial disparity in outcomes in endometrial cancer: could this be explained on a molecular level?[J]. Gynecol Oncol, 2006,102(3):440-446. |
| [4] | Srivastava SK, Ahmad A, Zubair H, et al. MicroRNAs in gynecological cancers: Small molecules with big implications[J]. Cancer Lett, 2017,407:123-138. |
| [5] | Robert JK, Maria LC, Herrington CS, et al. WHO classification of tumours of female reproductive organs[M]. Lyon, France: World Health Organization, 2014: 121-154. |
| [6] | 董颖, 周梅, 巴晓军, 等. 子宫内膜癌中DNA甲基转移酶3B的表达特点与临床意义[J]. 北京大学学报(医学版), 2016,48(5):788-794. |
| [7] | Chen T, Ding L, Shan G, et al. Prevalence and racial differences in pterygium: a cross-sectional study in Han and Uygur adults in Xinjiang, China[J]. Invest Ophthalmol Vis Sci, 2015,56(2):1109-1117. |
| [8] | Lee JW, Park YA, Choi JJ, et al. The expression of the miRNA-200 family in endometrial endometrioid carcinoma[J]. Gynecol Oncol, 2011,120(1):56-62. |
| [9] | Ratner ES, Tuck D, Richter C, et al. MicroRNA signatures differentiate uterine cancer tumor subtypes[J]. Gynecol Oncol, 2010,118(3):251-257. |
| [10] | Chung TK, Cheung TH, Huen NY, et al. Dysregulated micro-RNAs and their predicted targets associated with endometrioid endometrial adenocarcinoma in Hong Kong women[J]. Int J Can-cer, 2009,124(6):1358-1365. |
| [11] | 刘霞, 夏伟, 代荫梅, 等. 子宫内膜腺癌组织中miRNA的差异表达[J]. 中华医学杂志, 2009,89(19):1365-1367. |
| [12] | Wu W, Lin Z, Zhuang Z, et al. Expression profile of mammalian microRNAs in endometrioid adenocarcinoma[J]. Eur J Cancer Prev, 2009,18(1):50-55. |
| [13] | Hiroki E, Akahira J, Suzuki F, et al. Changes in microRNA expression levels correlate with clinicopathological features and prognoses in endometrial serous adenocarcinomas[J]. Cancer Sci, 2010,101(1):241-249. |
| [14] |
Devor EJ, Hovey AM, Goodheart MJ, et al. microRNA expression profiling of endometrial endometrioid adenocarcinomas and serous adenocarcinomas reveals profiles containing shared, unique and differentiating groups of microRNAs[J]. Oncol Rep, 2011,26(4):995-1002.
pmid: 21725615 |
| [15] |
Snowdon J, Zhang X, Childs T, et al. The microRNA-200 family is upregulated in endometrial carcinoma[J]. PLoS One, 2011,6(8):e22828.
doi: 10.1371/journal.pone.0022828 pmid: 21897839 |
| [16] |
Torres A, Torres K, Pesci A, et al. Diagnostic and prognostic significance of miRNA signatures in tissues and plasma of endome-trioid endometrial carcinoma patients[J]. Int J Cancer, 2013,132(7):1633-1645.
doi: 10.1002/ijc.27840 pmid: 22987275 |
| [17] |
Bauer KM, Hummon AB. Effects of the miR-143/-145 microRNA cluster on the colon cancer proteome and transcriptome[J]. J Proteome Res, 2012,11(9):4744-4754.
doi: 10.1021/pr300600r |
| [18] |
Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets[J]. Proc Natl Acad Sci USA, 2006,103(7):2257-2261.
doi: 10.1073/pnas.0510565103 pmid: 16461460 |
| [19] |
Zhang X, Dong Y, Ti H, et al. Down-regulation of miR-145 and miR-143 might be associated with DNA methyltransferase 3B overexpression and worse prognosis in endometrioid carcinomas[J]. Hum Pathol, 2013,44(11):2571-2580.
doi: 10.1016/j.humpath.2013.07.002 |
| [1] | Shuai LIU,Lei LIU,Zhuo LIU,Fan ZHANG,Lulin MA,Xiaojun TIAN,Xiaofei HOU,Guoliang WANG,Lei ZHAO,Shudong ZHANG. Clinical treatment and prognosis of adrenocortical carcinoma with venous tumor thrombus [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 624-630. |
| [2] | Fan SHU,Yichang HAO,Zhanyi ZHANG,Shaohui DENG,Hongxian ZHANG,Lei LIU,Guoliang WANG,Xiaojun TIAN,Lei ZHAO,Lulin MA,Shudong ZHANG. Functional and oncologic outcomes of partial nephrectomy for cystic renal cell carcinoma: A single-center retrospective study [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 667-672. |
| [3] | Zezhen ZHOU,Shaohui DENG,Ye YAN,Fan ZHANG,Yichang HAO,Liyuan GE,Hongxian ZHANG,Guoliang WANG,Shudong ZHANG. Predicting the 3-year tumor-specific survival in patients with T3a non-metastatic renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 673-679. |
| [4] | Junqi SU,Xiaoying WANG,Zhiqiang SUN. Establishment and verification of a prognostic nomogram for survival of tongue squamous cell carcinoma patients who underwent cervical dissection [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 120-130. |
| [5] | Wen-gen LI,Xiao-dong GU,Rui-qiang WENG,Su-dong LIU,Chao CHEN. Expression and clinical significance of plasma exosomal miR-34-5p and miR-142-3p in systemic sclerosis [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1022-1027. |
| [6] | Yun-chong LIU,Zong-long WU,Li-yuan GE,Tan DU,Ya-qian WU,Yi-meng SONG,Cheng LIU,Lu-lin MA. Mechanism of nuclear protein 1 in the resistance to axitinib in clear cell renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 781-792. |
| [7] | Dong LAN,Zhuo LIU,Yu-xuan LI,Guo-liang WANG,Xiao-jun TIAN,Lu-lin MA,Shu-dong ZHANG,Hong-xian ZHANG. Risk factors for massive hemorrhage after radical nephrectomy and removal of venous tumor thrombus [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 825-832. |
| [8] | Shang XIE,Zhi-gang CAI,Xiao-feng SHAN. Application value of whole exon sequencing and immune related indicators in the precision treatment of oral squamous cell carcinoma [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 697-701. |
| [9] | Han LU,Jian-yun ZHANG,Rong YANG,Le XU,Qing-xiang LI,Yu-xing GUO,Chuan-bin GUO. Clinical factors affecting the prognosis of lower gingival squamous cell carcinoma [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 702-707. |
| [10] | Yun-yi XU,Zheng-zheng SU,Lin-mao ZHENG,Meng-ni ZHANG,Jun-ya TAN,Ya-lan YANG,Meng-xin ZHANG,Miao XU,Ni CHEN,Xue-qin CHEN,Qiao ZHOU. Read-through circular RNA rt-circ-HS promotes hypoxia inducible factor 1α expression and renal carcinoma cell proliferation, migration and invasiveness [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 217-227. |
| [11] | Jing YANG,Juan DU,Yu-xiang WANG,Cong-rong LIU. Activation of JAK/STAT in ovarian high-grade serous cancers and its prognostic significance [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 270-275. |
| [12] | Qi SHEN,Yi-xiao LIU,Qun HE. Mucinous tubular and spindle cell carcinoma of kidney: Clinicopathology and prognosis [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 276-282. |
| [13] | Dong LI,Ji-ting DI,Yan XIONG. Consistency comparison of programmed cell death 1-ligand 1 in different immuno-histochemical staining methods [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 339-342. |
| [14] | Bo-han NING,Qing-xia ZHANG,Hui YANG,Ying DONG. Endometrioid adenocarcinoma with proliferated stromal cells, hyalinization and cord-like formations: A case report [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 366-369. |
| [15] | Fang CAO,Ming ZHONG,Cong-rong LIU. Uterine POLE mutant endometrioid carcinoma combined with human papilloma virus-associated cervical adenocarcinoma: A case report and literature review [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 370-374. |
|
||