Journal of Peking University (Health Sciences) ›› 2020, Vol. 52 ›› Issue (3): 564-569. doi: 10.19723/j.issn.1671-167X.2020.03.025
Previous Articles Next Articles
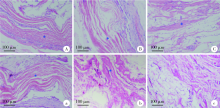
Biodegradation properties of multi-laminated small intestinal submucosa
Wei-yi WU,Bo-wen LI,Yu-hua LIU( ),Xin-zhi WANG
),Xin-zhi WANG
- Department of Prosthodontics, Peking University School and Hospital of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
CLC Number:
- R318.021
| [1] | Lopez-Martinez F, Gomez Moreno G, Olivares-Ponce P, et al. Implants failures related to endodontic treatment. An observational retrospective study[J]. Clin Oral Implants Res, 2015,26(9):992-995. |
| [2] | Palachur D, Prabhakara Rao KV, Murthy KR, et al. A comparative evaluation of bovine-derived xenograft (Bio-Oss collagen) and type Ⅰ collagen membrane (Bio-Gide) with bovine-derived xenograft (Bio-Oss collagen) and fibrin fibronectin sealing system (tisseel) in the treatment of intrabony defects: A clinico-radiographic study[J]. J Indian Soc Periodontol, 2014,18(3):336-343. |
| [3] | Amoian B, Moudi E, Majidi MS, et al. A histologic, histomorphometric, and radiographic comparison between two complexes of cenoboen/cenomembrane and bio-oss/Bio-Gide in lateral ridge augmentation: A clinical trial[J]. Dent Res J (Isfahan), 2016,13(5):446-453. |
| [4] | Oortgiesen DA, Plachokova AS, Geenen C, et al. Alkaline phosphatase immobilization onto Bio-Gide® and Bio-Oss® for periodontal and bone regeneration [J]. J Clin Periodontol, 2012,39(6):546-555. |
| [5] | 詹雅琳, 胡文杰, 甄敏, 等. 去蛋白牛骨基质与可吸收胶原膜的磨牙拔牙位点保存效果影像学评价[J]. 北京大学学报(医学版), 2015,47(1):19-26. |
| [6] | Strietzel FP, Khongkhunthian P, Khattiya R, et al. Healing pattern of bone defects covered by different membrane types: a histologic study in the porcine mandible[J]. J Biomed Mater Res B Appl Biomater, 2006,78(1):35-46. |
| [7] |
Owens KW, Yukna RA. Collagen membrane resorption in dogs: A comparative study[J]. Implant Dent, 2001,10(1):49-58.
doi: 10.1097/00008505-200101000-00016 pmid: 11307648 |
| [8] | 吴唯伊, 李博文, 刘玉华, 等. 猪小肠黏膜下层可吸收膜性能及修复骨缺损的效果评价[C]. 中华口腔医学会口腔修复学专业委员会.第十一次全国口腔修复学学术会议论文汇编.南京: 2017. |
| [9] | Olaechea A, Doza-Azpur GM, Valdivia E, et al. Biodegradation of three different collagen membranes: A histological study[J]. Journal of Osseointegration, 2016,8(2):15-19. |
| [10] |
Pilipchuk SP, Plonka AB, Monje A, et al. Tissue engineering for bone regeneration and osseointegration in the oral cavity[J]. Dent Mater, 2015,31(4):317-338.
pmid: 25701146 |
| [11] |
Mcallister BS, Haghighat K. Bone augmentation techniques[J]. J Periodontol, 2007,78(3):377-396.
pmid: 17335361 |
| [12] |
Bresaola MD, Matsumoto MA, Zahoui A, et al. Influence of rapid- and slow-rate resorption collagen membrane in maxillary sinus augmentation[J]. Clin Oral Implants Res, 2017,28(3):320-326.
doi: 10.1111/clr.12801 pmid: 26916561 |
| [13] |
Moses O, Vitrial D, Aboodi G, et al. Biodegradation of three different collagen membranes in the rat calvarium: A comparative study[J]. J Periodontol, 2008,79(5):905-911.
pmid: 18454670 |
| [14] |
Sheikh Z, Hamdan N, Ikeda Y, et al. Natural graft tissues and synthetic biomaterials for periodontal and alveolar bone reconstructive applications: A review[J]. Biomater Res, 2017,21:9.
doi: 10.1186/s40824-017-0095-5 pmid: 28593053 |
| [15] | Bozkurt A, Apel C, Sellhaus B, et al. Differences in degradation behavior of two non-cross-linked collagen barrier membranes: An in vitro and in vivo study[J]. Clin Oral Implants Res, 2014,25(12):1403-1411. |
| [16] |
Bozkurt A, Lassner F, O’dey D, et al. The role of microstructured and interconnected pore channels in a collagen-based nerve guide on axonal regeneration in peripheral nerves[J]. Biomaterials, 2012,33(5):1363-1375.
doi: 10.1016/j.biomaterials.2011.10.069 pmid: 22082619 |
| [17] | Bozkurt A, Deumens R, Beckmann C, et al. In vitro cell alignment obtained with a schwann cell enriched microstructured nerve guide with longitudinal guidance channels[J]. Biomaterials, 2009,30(2):169-179. |
| [18] |
Verissimo DM, Leitao RF, Ribeiro RA, et al. Polyanionic collagen membranes for guided tissue regeneration: Effect of progressive glutaraldehyde cross-linking on biocompatibility and degradation[J]. Acta Biomater, 2010,6(10):4011-4018.
pmid: 20433958 |
| [19] | Glynn JJ, Polsin EG, Hinds MT. Crosslinking decreases the hemocompatibility of decellularized, porcine small intestinal submucosa[J]. Acta Biomater, 2015,14:96-103. |
| [20] | Moses O, Pitaru S, Artzi Z, et al. Healing of dehiscence-type defects in implants placed together with different barrier membranes: A comparative clinical study[J]. Clin Oral Implants Res, 2005,16(2):210-219. |
| [21] |
Valentin JE, Stewart-Akers AM, Gilbert TW, et al. Macrophage participation in the degradation and remodeling of extracellular matrix scaffolds[J]. Tissue Eng Part A, 2009,15(7):1687-1694.
pmid: 19125644 |
| [22] |
Valentin JE, Badylak JS, Mccabe GP, et al. Extracellular matrix bioscaffolds for orthopaedic applications. A comparative histologic study[J]. J Bone Joint Surg Am, 2006,88(12):2673-2686.
pmid: 17142418 |
| [23] |
Bai H, Wang D, Delattre B, et al. Biomimetic gradient scaffold from ice-templating for self-seeding of cells with capillary effect[J]. Acta Biomater, 2015,20:113-119.
doi: 10.1016/j.actbio.2015.04.007 pmid: 25871536 |
| [24] |
Badylak SF, Gilbert TW. Immune response to biologic scaffold materials[J]. Semin Immunol, 2008,20(2):109-116.
pmid: 18083531 |
| [25] |
Yan HJ, Casalini T, Hulsart-Billstrom G, et al. Synthetic design of growth factor sequestering extracellular matrix mimetic hydrogel for promoting in vivo bone formation[J]. Biomaterials, 2018,161:190-202.
doi: 10.1016/j.biomaterials.2018.01.041 pmid: 29421555 |
| [26] | Rothamel D, Benner M, Fienitz T, et al. Biodegradation pattern and tissue integration of native and cross-linked porcine collagen soft tissue augmentation matrices: An experimental study in the rat[J]. Head Face Med, 2014(10):10. |
| [27] |
Siar CH, Toh CG, Romanos G, et al. Subcutaneous reactions and degradation characteristics of collagenous and noncollagenous membranes in a macaque model[J]. Clin Oral Implants Res, 2011,22(1):113-120.
doi: 10.1111/j.1600-0501.2010.01970.x pmid: 20678135 |
| [28] |
Olde Damink LH, Dijkstra PJ, Van Luyn MJ, et al. In vitro degradation of dermal sheep collagen cross-linked using a water-soluble carbodiimide[J]. Biomaterials, 1996,17(7):679-684.
doi: 10.1016/0142-9612(96)86737-8 pmid: 8672629 |
| [29] |
Li J, Ren N, Qiu J, et al. Carbodiimide crosslinked collagen from porcine dermal matrix for high-strength tissue engineering scaffold[J]. Int J Biol Macromol, 2013,61:69-74.
doi: 10.1016/j.ijbiomac.2013.06.038 pmid: 23820178 |
| [30] | 闫建伟. 牙种植引导骨再生心包胶原膜的制备及理化性能研究[D]. 济南: 山东大学, 2017. |
| [31] |
Kozlovsky A, Aboodi G, Moses O, et al. Bio-degradation of a resorbable collagen membrane (Bio-Gide) applied in a double-layer technique in rats[J]. Clin Oral Implants Res, 2009,20(10):1116-1123.
doi: 10.1111/j.1600-0501.2009.01740.x pmid: 19719734 |
| [32] |
Gilbert TW, Stewart-Akers AM, Simmons-Byrd A, et al. Degradation and remodeling of small intestinal submucosa in canine achilles tendon repair[J]. J Bone Joint Surg Am, 2007,89(3):621-630.
doi: 10.2106/JBJS.E.00742 pmid: 17332112 |
| [33] |
Record RD, Hillegonds D, Simmons C, et al. In vivo degradation of c-14-labeled small intestinal submucosa (sis) when used for urinary bladder repair[J]. Biomaterials, 2001,22(19):2653-2659.
doi: 10.1016/S0142-9612(01)00007-2 |
| [34] | Mewaldt R, Shi L, Carson D. Enzymatic degradation study of single layer and multi-layer small intestine submucosa (sis) matrices[J]. Wound Repair Regen, 2011,19(2):A39. |
| [1] | Han ZHAO,Yan WEI,Xuehui ZHANG,Xiaoping YANG,Qing CAI,Chengyun NING,Mingming XU,Wenwen LIU,Ying HUANG,Ying HE,Yaru GUO,Shengjie JIANG,Yunyang BAI,Yujia WU,Yusi GUO,Xiaona ZHENG,Wenjing LI,Xuliang DENG. Bionic design, preparation and clinical translation of oral hard tissue restorative materials [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 4-8. |
| [2] | Xiaoying CHEN,Yi ZHANG,Yuke LI,Lin TANG,Yuhua LIU. Effects of different polymers on biomimetic mineralization of small intestine submucosal scaffolds [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 17-24. |
| [3] | Yu-ke LI,Mei WANG,Lin TANG,Yu-hua LIU,Xiao-ying CHEN. Effect of pH on the chelation between strontium ions and decellularized small intestinal submucosal sponge scaffolds [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 44-51. |
| [4] | Yi DENG,Yi ZHANG,Bo-wen LI,Mei WANG,Lin TANG,Yu-hua LIU. Effects of different crosslinking treatments on the properties of decellularized small intestinal submucosa porous scaffolds [J]. Journal of Peking University (Health Sciences), 2022, 54(3): 557-564. |
| [5] | YOU Peng-yue,LIU Yu-hua,WANG Xin-zhi,WANG Si-wen,TANG Lin. Biocompatibility and effect on bone formation of a native acellular porcine pericardium: Results of in vitro and in vivo [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 776-784. |
| [6] | WANG Si-wen,YOU Peng-yue,LIU Yu-hua,WANG Xin-zhi,TANG Lin,WANG Mei. Efficacy of two barrier membranes and deproteinized bovine bone mineral on bone regeneration in extraction sockets: A microcomputed tomographic study in dogs [J]. Journal of Peking University (Health Sciences), 2021, 53(2): 364-370. |
| [7] | Mei WANG, Bo-wen LI, Si-wen WANG, Yu-hua LIU. Preparation and osteogenic effect study of small intestinal submucosa sponge [J]. Journal of Peking University (Health Sciences), 2020, 52(5): 952-958. |
| [8] | Dong SHI,Jie CAO,Shi-ai DAI,Huan-xin MENG. Short-term outcome of regenerative surgery treating peri-implantitis [J]. Journal of Peking University(Health Sciences), 2020, 52(1): 58-63. |
| [9] | Bo-wen LI,Wei-yi WU,Lin TANG,Yi ZHANG,Yu-hua LIU. Barrier effect of improved porcine small intestinal submucosa absorbable membrane on early healing of mandibular defects in rabbits [J]. Journal of Peking University(Health Sciences), 2019, 51(5): 887-892. |
| [10] | Chen LIANG,Wei-yu ZHANG,Hao HU,Qi WANG,Zhi-wei FANG,Ke-xin XU. Comparison of effectiveness and complications between two different methods of augmentation cystoplasty [J]. Journal of Peking University(Health Sciences), 2019, 51(2): 293-297. |
| [11] | ZHAN Ya-lin, HU Wen-jie, XU Tao, ZHEN Min, LU Rui-fang. Histomorphometric evaluation of ridge preservation after molar tooth extraction [J]. Journal of Peking University(Health Sciences), 2017, 49(1): 169-175. |
| [12] | SONG Yang, WANG Xiao-fei, WANG Yu-guang, SUN Yu-chun, LV Pei-jun. Osteogenesis of human adipose-derived mesenchymal stem cells-biomaterial mixture in vivo after 3D bio-printing [J]. Journal of Peking University(Health Sciences), 2016, 48(1): 45-50. |
| [13] | ZHAN Ya-Lin, HU Wen-Jie, ZHEN Min, Xu-Tao, Lu-Rui-Fang. Radiographic evaluation of ridge preservation after molar tooth extraction: a controlled clinical trial [J]. Journal of Peking University(Health Sciences), 2015, 47(1): 19-26. |
|
||