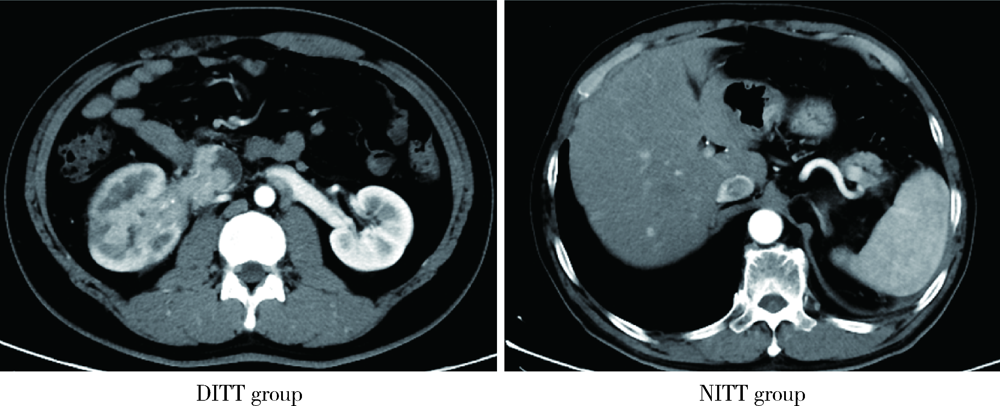
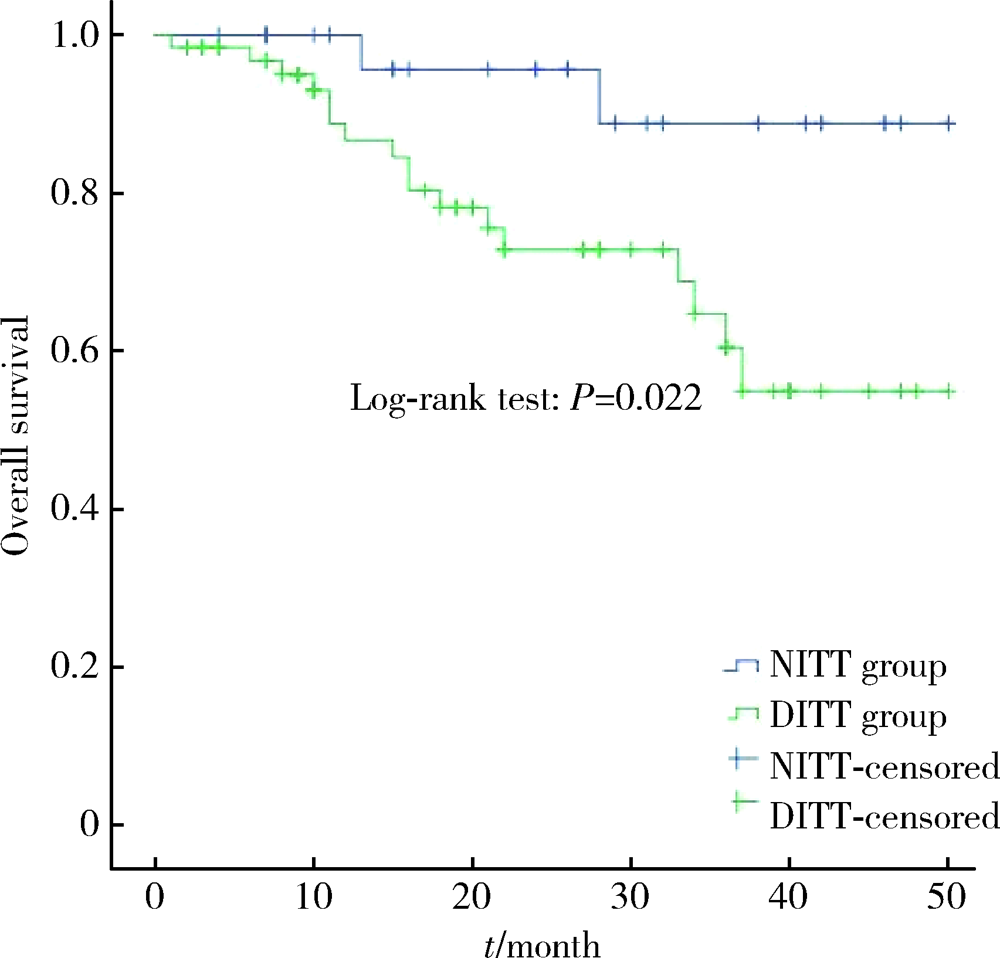
Journal of Peking University (Health Sciences) ›› 2021, Vol. 53 ›› Issue (4): 665-670. doi: 10.19723/j.issn.1671-167X.2021.04.007
Previous Articles Next Articles
Influence of deep invasive tumor thrombus on the surgical treatment and prognosis of patients with non-metastatic renal cell carcinoma complicated with venous tumor thrombus
ZHAO Xun,YAN Ye,HUANG Xiao-juan,DONG Jing-han,LIU Zhuo,ZHANG Hong-xian,LIU Cheng( ),MA Lu-lin(
),MA Lu-lin( )
)
- Department of Urology, Peking University Third Hospital, Beijing 100191, China
CLC Number:
- R737.11
| [1] |
Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018 [J]. Eur J Cancer, 2018, 103:356-387.
doi: S0959-8049(18)30955-9 pmid: 30100160 |
| [2] |
Lardas M, Stewart F, Scrimgeour D, et al. Systematic review of surgical management of nonmetastatic renal cell carcinoma with vena caval thrombus [J]. Eur Urol, 2016, 70(2):265-280.
doi: 10.1016/j.eururo.2015.11.034 |
| [3] |
Quencer KB, Friedman T, Sheth R, et al. Tumor thrombus: incidence, imaging, prognosis and treatment [J]. Cardiovasc Diagn Ther, 2017, 7(Suppl 3):S165-S177.
doi: 10.21037/cdt |
| [4] |
González J, Gorin MA, Garcia-Roig M, et al. Inferior vena cava resection and reconstruction: Technical considerations in the surgical management of renal cell carcinoma with tumor thrombus [J]. Urol Oncol, 2014, 32(1): 34.e19-26.
doi: 10.1016/j.urolonc.2013.01.004 |
| [5] |
Adams LC, Ralla B, Bender YY, et al. Renal cell carcinoma with venous extension: prediction of inferior vena cava wall invasion by MRI [J]. Cancer Imaging, 2018, 18(1):17.
doi: 10.1186/s40644-018-0150-z pmid: 29724245 |
| [6] |
Psutka SP, Boorjian SA, Thompson RH, et al. Clinical and radiographic predictors of the need for inferior vena cava resection during nephrectomy for patients with renal cell carcinoma and caval tumour thrombus [J]. BJU Int, 2015, 116(3):388-396.
doi: 10.1111/bju.13005 pmid: 25430786 |
| [7] | Liu Z, Li L, Hong P, et al. A predictive model for tumor invasion of the inferior vena cava wall using multimodal imaging in patients with renal cell carcinoma and inferior vena cava tumor thrombus [J]. Biomed Res Int, 2020, 2020:9530618. |
| [8] |
Li QY, Li N, Huang QB, et al. Contrast-enhanced ultrasound in detecting wall invasion and differentiating bland from tumor thrombus during robot-assisted inferior vena cava thrombectomy for renal cell carcinoma [J]. Cancer Imaging, 2019, 19(1):79.
doi: 10.1186/s40644-019-0265-x |
| [9] |
Heng DY, Xie W, Regan MM, et al. External validation and comparison with other models of the international metastatic renal-cell carcinoma database consortium prognostic model: A population-based study [J]. Lancet Oncol, 2013, 14(2):141-148.
doi: 10.1016/S1470-2045(12)70559-4 |
| [10] |
Xiao R, Xu C, He W, et al. Preoperative anaemia and thrombocytosis predict adverse prognosis in non-metastatic renal cell carcinoma with tumour thrombus [J]. BMC Urol, 2021, 21(1):31.
doi: 10.1186/s12894-021-00796-6 |
| [11] |
Du S, Huang Q, Yu H, et al. Initial series of robotic segmental inferior vena cava resection in left renal cell carcinoma with caval tumor thrombus [J]. Urology, 2020, 142:125-132.
doi: 10.1016/j.urology.2020.03.053 |
| [12] |
Gu L, Li H, Wang Z, et al. A systematic review and meta-analysis of clinicopathologic factors linked to oncologic outcomes for renal cell carcinoma with tumor thrombus treated by radical nephrectomy with thrombectomy [J]. Cancer Treat Rev, 2018, 69:112-120.
doi: 10.1016/j.ctrv.2018.06.014 |
| [13] |
Rodriguez Faba O, Linares E, Tilki D, et al. Impact of microscopic wall invasion of the renal vein or inferior vena cava on cancer-specific survival in patients with renal cell carcinoma and tumor thrombus: A multi-institutional analysis from the International Renal Cell Carcinoma-Venous Thrombus Consortium [J]. Eur Urol Focus, 2018, 4(3):435-441.
doi: S2405-4569(17)30018-4 pmid: 28753848 |
| [14] |
Wang H, Li X, Huang Q, et al. Prognostic role of bland thrombus in patients treated with resection of renal cell carcinoma with infe-rior vena cava tumor thrombus [J]. Urol Oncol, 2021, 39(5): 302.e1-e7.
doi: 10.1016/j.urolonc.2021.02.005 |
| [1] | Junyong OU,Kunming NI,Lulin MA,Guoliang WANG,Ye YAN,Bin YANG,Gengwu LI,Haodong SONG,Min LU,Jianfei YE,Shudong ZHANG. Prognostic factors of patients with muscle invasive bladder cancer with intermediate-to-high risk prostate cancer [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 582-588. |
| [2] | Kewei CHEN,Zhuo LIU,Shaohui DENG,Fan ZHANG,Jianfei YE,Guoliang WANG,Shudong ZHANG. Clinical diagnosis and treatment of renal angiomyolipoma with inferior vena cava tumor thrombus [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 617-623. |
| [3] | Shuai LIU,Lei LIU,Zhuo LIU,Fan ZHANG,Lulin MA,Xiaojun TIAN,Xiaofei HOU,Guoliang WANG,Lei ZHAO,Shudong ZHANG. Clinical treatment and prognosis of adrenocortical carcinoma with venous tumor thrombus [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 624-630. |
| [4] | Jie YANG,Jieli FENG,Shudong ZHANG,Lulin MA,Qing ZHENG. Clinical effects of transesophageal echocardiography in different surgical methods for nephrectomy combined with Mayo Ⅲ-Ⅳ vena tumor thrombectomy [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 631-635. |
| [5] | Binshuai WANG,Min QIU,Qianjin ZHANG,Maofeng TIAN,Lei LIU,Guoliang WANG,Min LU,Xiaojun TIAN,Shudong ZHANG. Experience in diagnosis and treatment of 6 cases of renal Ewing's sarcoma with venous thrombus [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 636-639. |
| [6] | Le YU,Shaohui DENG,Fan ZHANG,Ye YAN,Jianfei YE,Shudong ZHANG. Clinicopathological characteristics and prognosis of multilocular cystic renal neoplasm of low malignant potential [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 661-666. |
| [7] | Fan SHU,Yichang HAO,Zhanyi ZHANG,Shaohui DENG,Hongxian ZHANG,Lei LIU,Guoliang WANG,Xiaojun TIAN,Lei ZHAO,Lulin MA,Shudong ZHANG. Functional and oncologic outcomes of partial nephrectomy for cystic renal cell carcinoma: A single-center retrospective study [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 667-672. |
| [8] | Zezhen ZHOU,Shaohui DENG,Ye YAN,Fan ZHANG,Yichang HAO,Liyuan GE,Hongxian ZHANG,Guoliang WANG,Shudong ZHANG. Predicting the 3-year tumor-specific survival in patients with T3a non-metastatic renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 673-679. |
| [9] | Yangyi FANG,Qiang LI,Zhigao HUANG,Min LU,Kai HONG,Shudong ZHANG. Well-differentiated papillary mesothelial tumour of the tunica vaginalis: A case report [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 741-744. |
| [10] | Yuanyuan ZENG,Yun XIE,Daonan CHEN,Ruilan WANG. Related factors of euthyroid sick syndrome in patients with sepsis [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 526-532. |
| [11] | Jian-bin LI,Meng-na LYU,Qiang CHI,Yi-lin PENG,Peng-cheng LIU,Rui WU. Early prediction of severe COVID-19 in patients with Sjögren’s syndrome [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1007-1012. |
| [12] | Yun-chong LIU,Zong-long WU,Li-yuan GE,Tan DU,Ya-qian WU,Yi-meng SONG,Cheng LIU,Lu-lin MA. Mechanism of nuclear protein 1 in the resistance to axitinib in clear cell renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 781-792. |
| [13] | Huan-rui LIU,Xiang PENG,Sen-lin LI,Xin GOU. Risk modeling based on HER-2 related genes for bladder cancer survival prognosis assessment [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 793-801. |
| [14] | Zi-xuan XUE,Shi-ying TANG,Min QIU,Cheng LIU,Xiao-jun TIAN,Min LU,Jing-han DONG,Lu-lin MA,Shu-dong ZHANG. Clinicopathologic features and prognosis of young renal tumors with tumor thrombus [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 802-811. |
| [15] | Dong LAN,Zhuo LIU,Yu-xuan LI,Guo-liang WANG,Xiao-jun TIAN,Lu-lin MA,Shu-dong ZHANG,Hong-xian ZHANG. Risk factors for massive hemorrhage after radical nephrectomy and removal of venous tumor thrombus [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 825-832. |
|
||