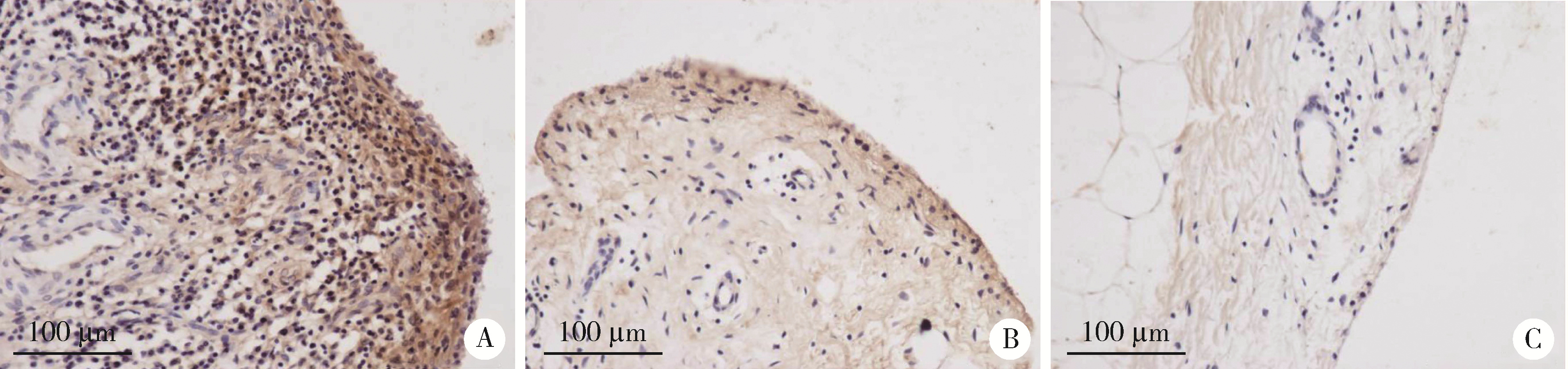
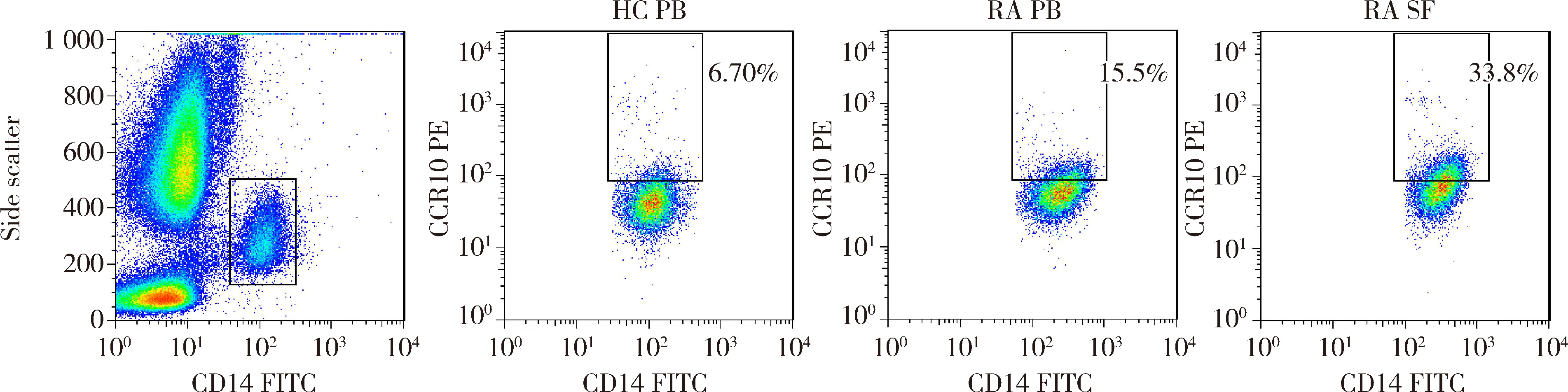
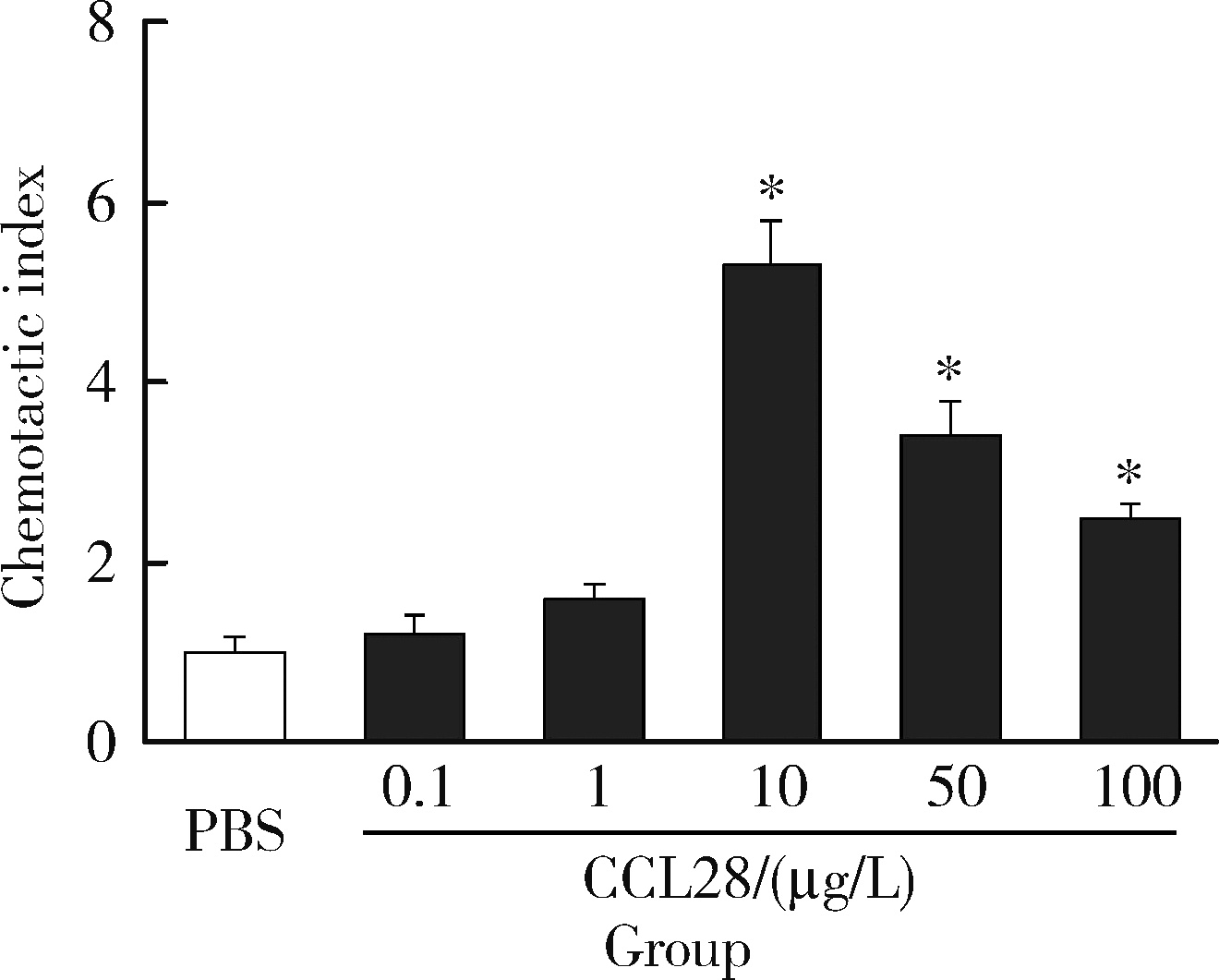
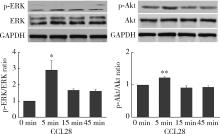
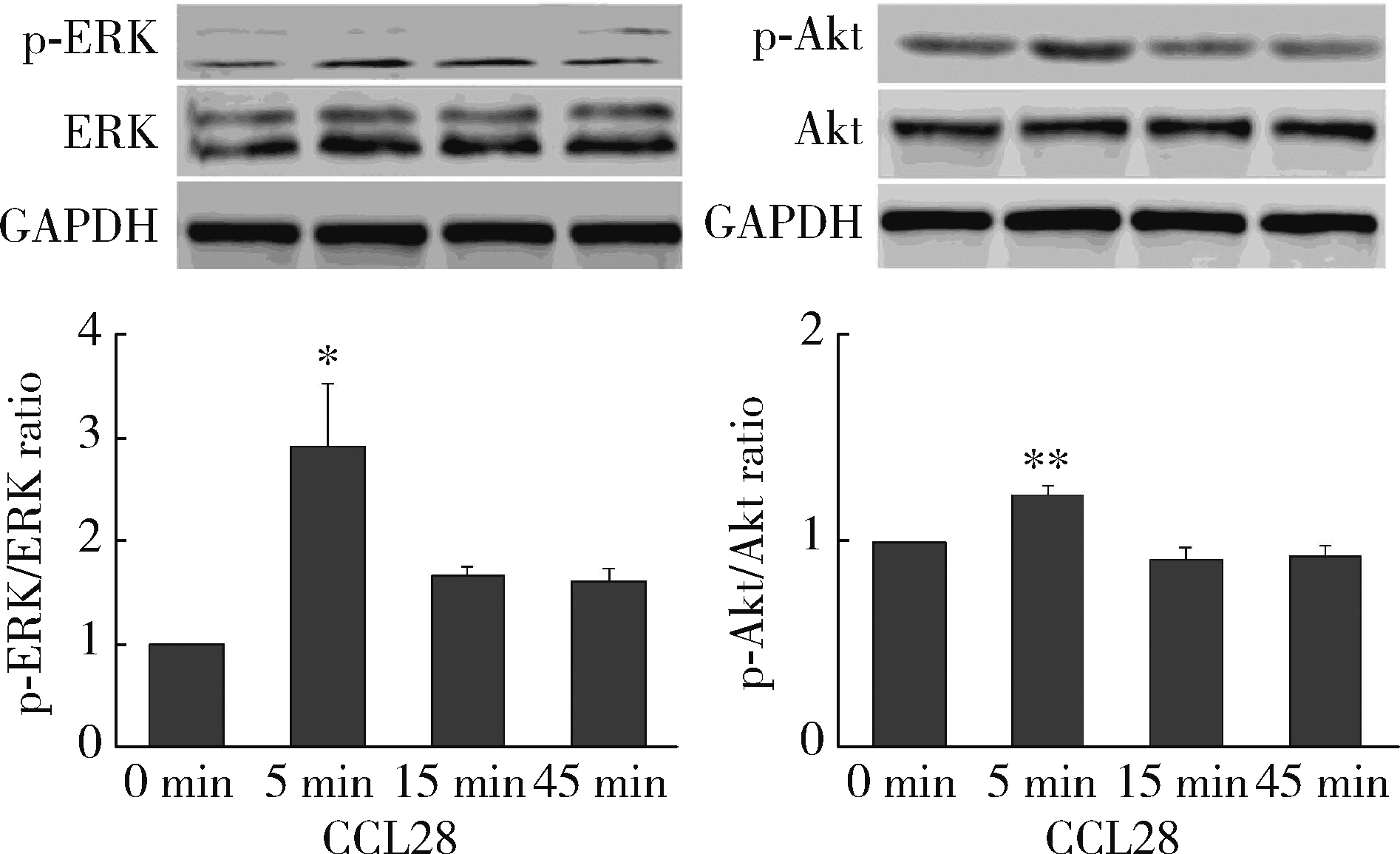
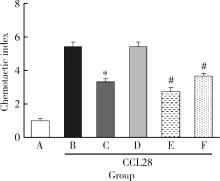
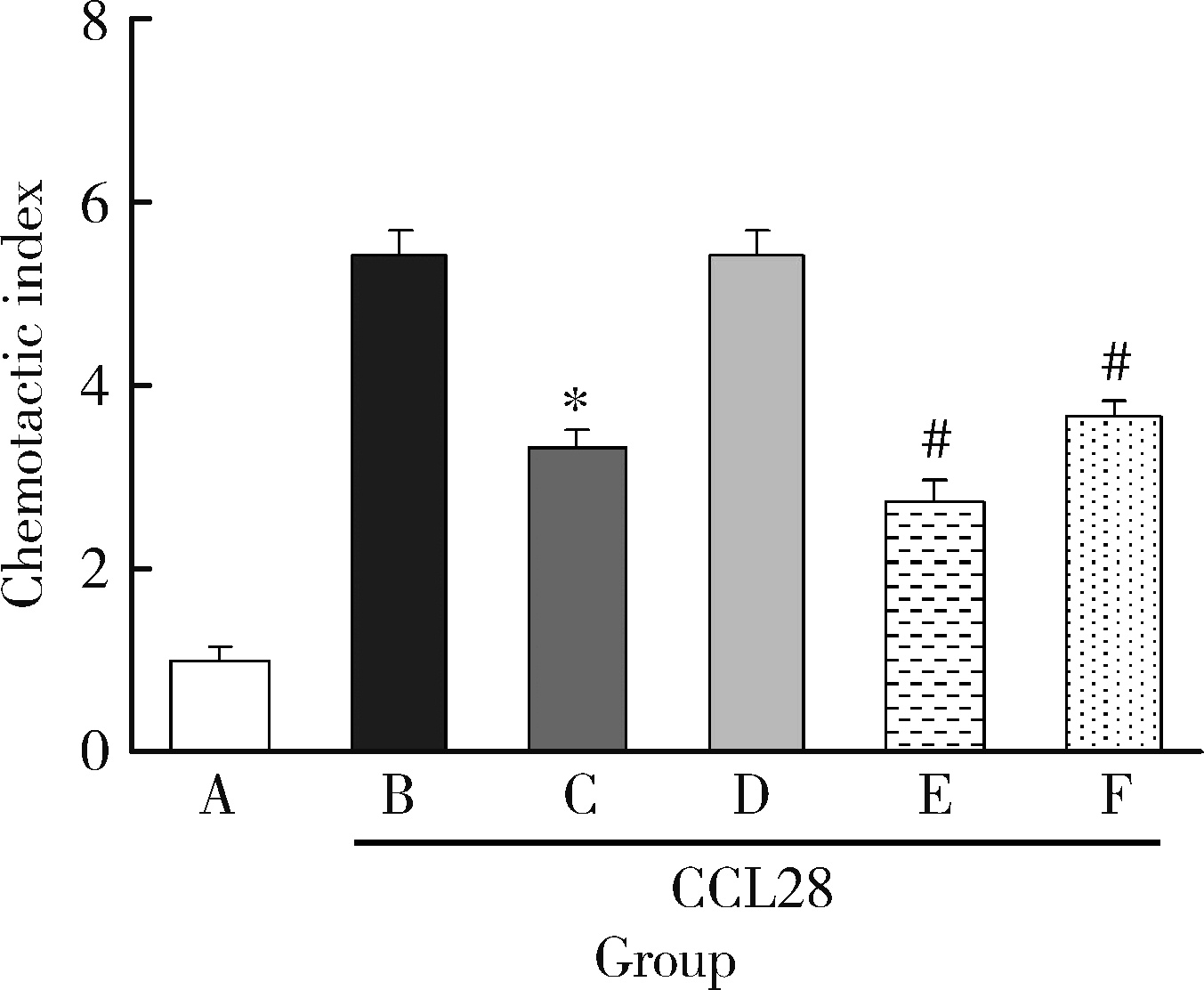
Journal of Peking University (Health Sciences) ›› 2022, Vol. 54 ›› Issue (6): 1074-1078. doi: 10.19723/j.issn.1671-167X.2022.06.003
Previous Articles Next Articles
Role of the CCL28-CCR10 pathway in monocyte migration in rheumatoid arthritis
Fang CHENG1,*( ),Shao-ying YANG2,Xing-xing FANG3,Xuan WANG3,Fu-tao ZHAO1,*(
),Shao-ying YANG2,Xing-xing FANG3,Xuan WANG3,Fu-tao ZHAO1,*( )
)
- 1. Department of Rheumatology and Immunology, Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 201999, China
2. Department of Rheumatology, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200001, China
3. Department of Rheumatology and Immunology, Tongji Hospital, Tongji University, Shanghai 200065, China
CLC Number:
- R593.22
| 1 |
Sparks JA . Rheumatoid arthritis[J]. Ann Intern Med, 2019, 170 (1): ITC1- ITC16.
doi: 10.7326/AITC201901010 |
| 2 |
Jang S , Kwon EJ , Lee JJ . Rheumatoid arthritis: Pathogenic roles of diverse immune cells[J]. Int J Mol Sci, 2022, 23 (2): 905.
doi: 10.3390/ijms23020905 |
| 3 |
Szekanecz Z , Koch AE . Successes and failures of chemokine-pathway targeting in rheumatoid arthritis[J]. Nat Rev Rheumatol, 2016, 12 (1): 5- 13.
doi: 10.1038/nrrheum.2015.157 |
| 4 |
Pan J , Kunkel EJ , Gosslar U , et al. A novel chemokine ligand for CCR10 and CCR3 expressed by epithelial cells in mucosal tissues[J]. J Immunol, 2000, 165 (6): 2943- 2949.
doi: 10.4049/jimmunol.165.6.2943 |
| 5 |
Wang W , Soto H , Oldham ER , et al. Identification of a novel chemokine (CCL28), which binds CCR10 (GPR2)[J]. J Biol Chem, 2000, 275 (29): 22313- 22323.
doi: 10.1074/jbc.M001461200 |
| 6 |
Mohan T , Deng L , Wang BZ . CCL28 chemokine: An anchoring point bridging innate and adaptive immunity[J]. Int Immuno-pharmacol, 2017, 51, 165- 170.
doi: 10.1016/j.intimp.2017.08.012 |
| 7 |
Chen Z , Kim SJ , Essani AB , et al. Characterising the expression and function of CCL28 and its corresponding receptor, CCR10, in RA pathogenesis[J]. Ann Rheum Dis, 2015, 74 (10): 1898- 1906.
doi: 10.1136/annrheumdis-2013-204530 |
| 8 |
Miyazaki Y , Nakayamada S , Kubo S , et al. Th22 cells promote osteoclast differentiation via production of IL-22 in rheumatoid arthritis[J]. Front Immunol, 2018, 9, 2901.
doi: 10.3389/fimmu.2018.02901 |
| 9 |
Aletaha D , Neogi T , Silman AJ , et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative[J]. Arthritis Rheum, 2010, 62 (9): 2569- 2581.
doi: 10.1002/art.27584 |
| 10 |
Rana AK , Li Y , Dang Q , et al. Monocytes in rheumatoid arthritis: Circulating precursors of macrophages and osteoclasts and, their heterogeneity and plasticity role in RA pathogenesis[J]. Int Immunopharmacol, 2018, 65, 348- 359.
doi: 10.1016/j.intimp.2018.10.016 |
| 11 |
Park J , Zhang X , Lee SK , et al. CCL28-induced RARβ expression inhibits oral squamous cell carcinoma bone invasion[J]. J Clin Invest, 2019, 129 (12): 5381- 5399.
doi: 10.1172/JCI125336 |
| 12 |
Facciabene A , Peng X , Hagemann IS , et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells[J]. Nature, 2011, 475 (7355): 226- 230.
doi: 10.1038/nature10169 |
| 13 |
Wu Q , Chen JX , Chen Y , et al. The chemokine receptor CCR10 promotes inflammation-driven hepatocarcinogenesis via PI3K/Akt pathway activation[J]. Cell Death Dis, 2018, 9 (2): 232.
doi: 10.1038/s41419-018-0267-9 |
| [1] | Dongwu LIU, Jie CHEN, Mingli GAO, Jing YU. Rheumatoid arthritis with Castleman-like histopathology in lymph nodes: A case report [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 928-931. |
| [2] | Huina HUANG,Jing ZHAO,Xiangge ZHAO,Ziran BAI,Xia LI,Guan WANG. Regulatory effect of lactate on peripheral blood CD4+ T cell subsets in patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 519-525. |
| [3] | Xiaofei TANG,Yonghong LI,Qiuling DING,Zhuo SUN,Yang ZHANG,Yumei WANG,Meiyi TIAN,Jian LIU. Incidence and risk factors of deep vein thrombosis in patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 279-283. |
| [4] | Xue ZOU,Xiao-juan BAI,Li-qing ZHANG. Effectiveness of tofacitinib combined with iguratimod in the treatment of difficult-to-treat moderate-to-severe rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1013-1021. |
| [5] | Qi WU,Yue-ming CAI,Juan HE,Wen-di HUANG,Qing-wen WANG. Correlation between dyslipidemia and rheumatoid arthritis associated interstitial lung disease [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 982-992. |
| [6] | Jing-feng ZHANG,Yin-ji JIN,Hui WEI,Zhong-qiang YAO,Jin-xia ZHAO. Correlation analysis between body mass index and clinical characteristics of rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 993-999. |
| [7] | Yin-ji JIN,Lin SUN,Jin-xia ZHAO,Xiang-yuan LIU. Significance of IgA isotype of anti-v-raf murine sarcoma viral oncogene homologue B1 antibody in rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 631-635. |
| [8] | Wen-xin CAI,Shi-cheng LI,Yi-ming LIU,Ru-yu LIANG,Jing LI,Jian-ping GUO,Fan-lei HU,Xiao-lin SUN,Chun LI,Xu LIU,Hua YE,Li-zong DENG,Ru LI,Zhan-guo LI. A cross-sectional study on the clinical phenotypes of rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1068-1073. |
| [9] | Rui LIU,Jin-xia ZHAO,Liang YAN. Clinical characteristics of patients with rheumatoid arthritis complicated with venous thrombosis of lower extremities [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1079-1085. |
| [10] | Jing-feng ZHANG,Yin-ji JIN,Hui WEI,Zhong-qiang YAO,Jin-xia ZHAO. Cross-sectional study on quality of life and disease activity of rheumatoid arthritis patients [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1086-1093. |
| [11] | GAO Chao,CHEN Li-hong,WANG Li,YAO Hong,HUANG Xiao-wei,JIA Yu-bo,LIU Tian. Validation of the Pollard’s classification criteria (2010) for rheumatoid arthritis patients with fibromyalgia [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 278-282. |
| [12] | ZHONG Hua,XU Li-ling,BAI Ming-xin,SU Yin. Effect of chemokines CXCL9 and CXCL10 on bone erosion in patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1026-1031. |
| [13] | LOU Xue,LIAO Li,LI Xing-jun,WANG Nan,LIU Shuang,CUI Ruo-mei,XU Jian. Methylation status and expression of TWEAK gene promoter region in peripheral blood of patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1020-1025. |
| [14] | LUO Liang,HUO Wen-gang,ZHANG Qin,LI Chun. Clinical characteristics and risk factors of rheumatoid arthritis with ulcerative keratitis [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1032-1036. |
| [15] | ZHANG Lu,HU Xiao-hong,CHEN Cheng,CAI Yue-ming,WANG Qing-wen,ZHAO Jin-xia. Analysis of cervical instability and clinical characteristics in treatment-naive rheumatoid arthritis patients [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1049-1054. |
|
||