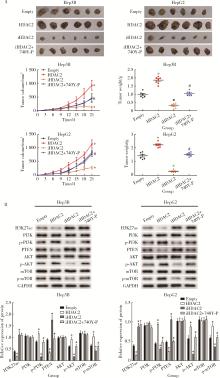
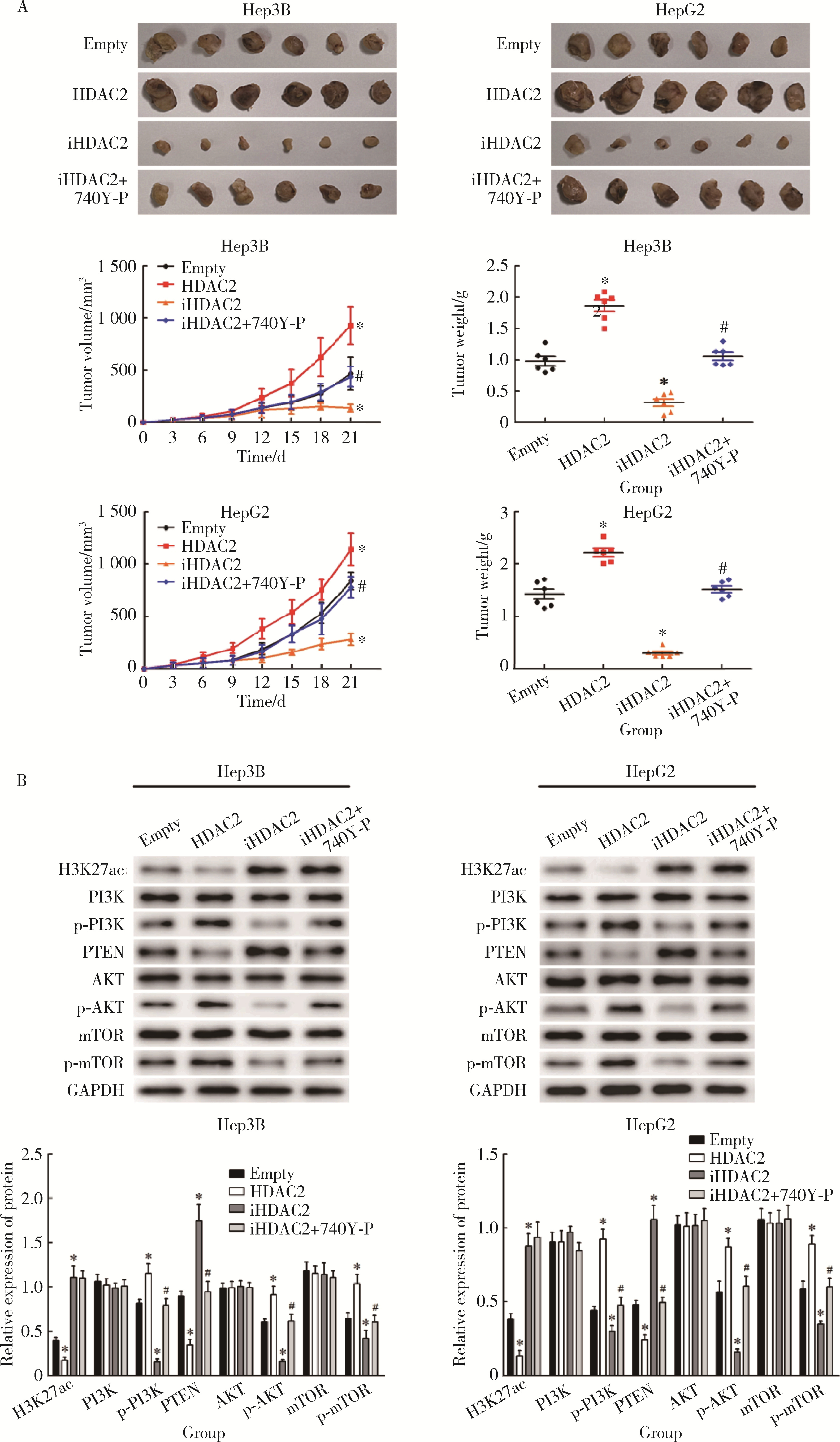
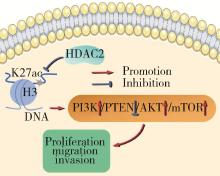
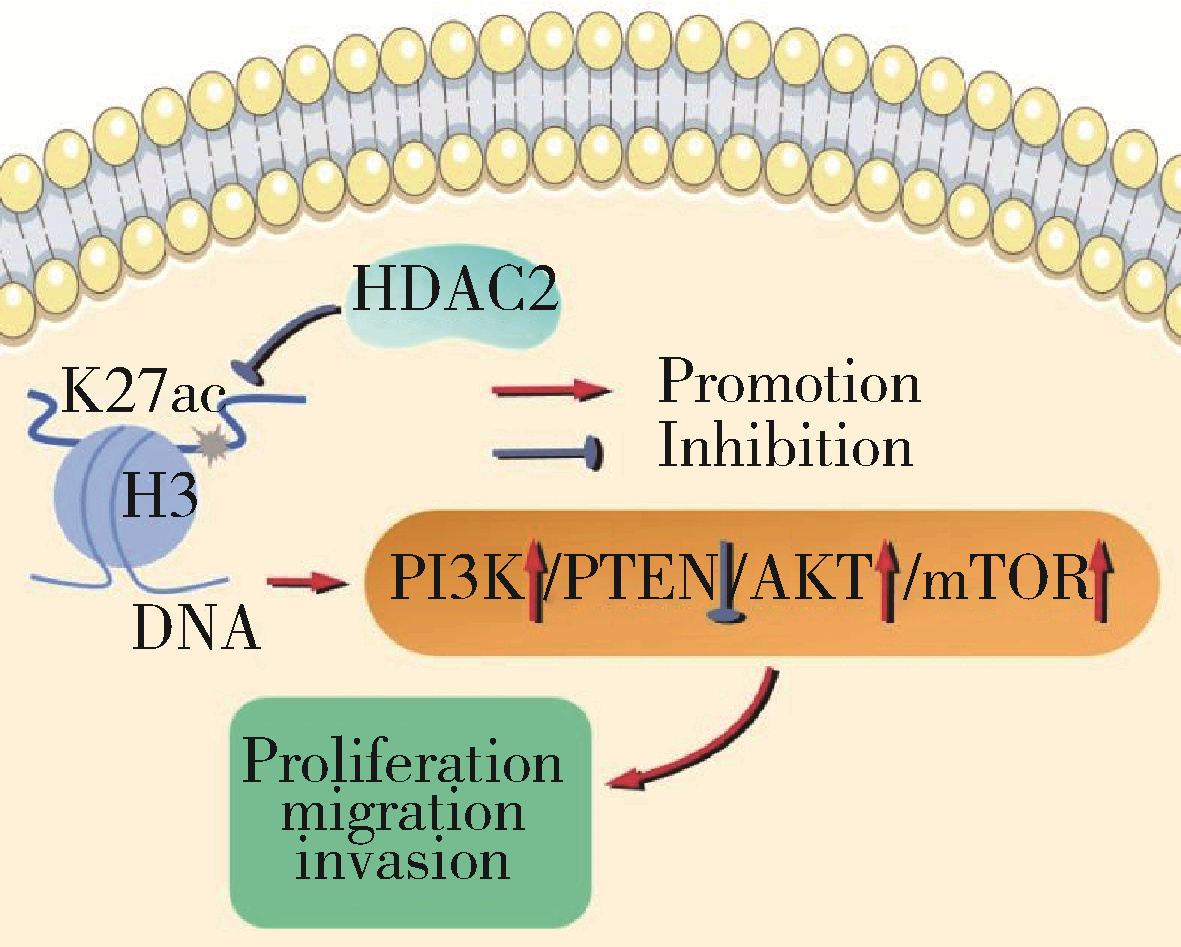
Journal of Peking University (Health Sciences) ›› 2025, Vol. 57 ›› Issue (5): 884-894. doi: 10.19723/j.issn.1671-167X.2025.05.012
Previous Articles Next Articles
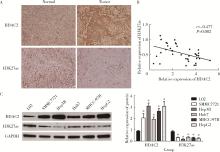
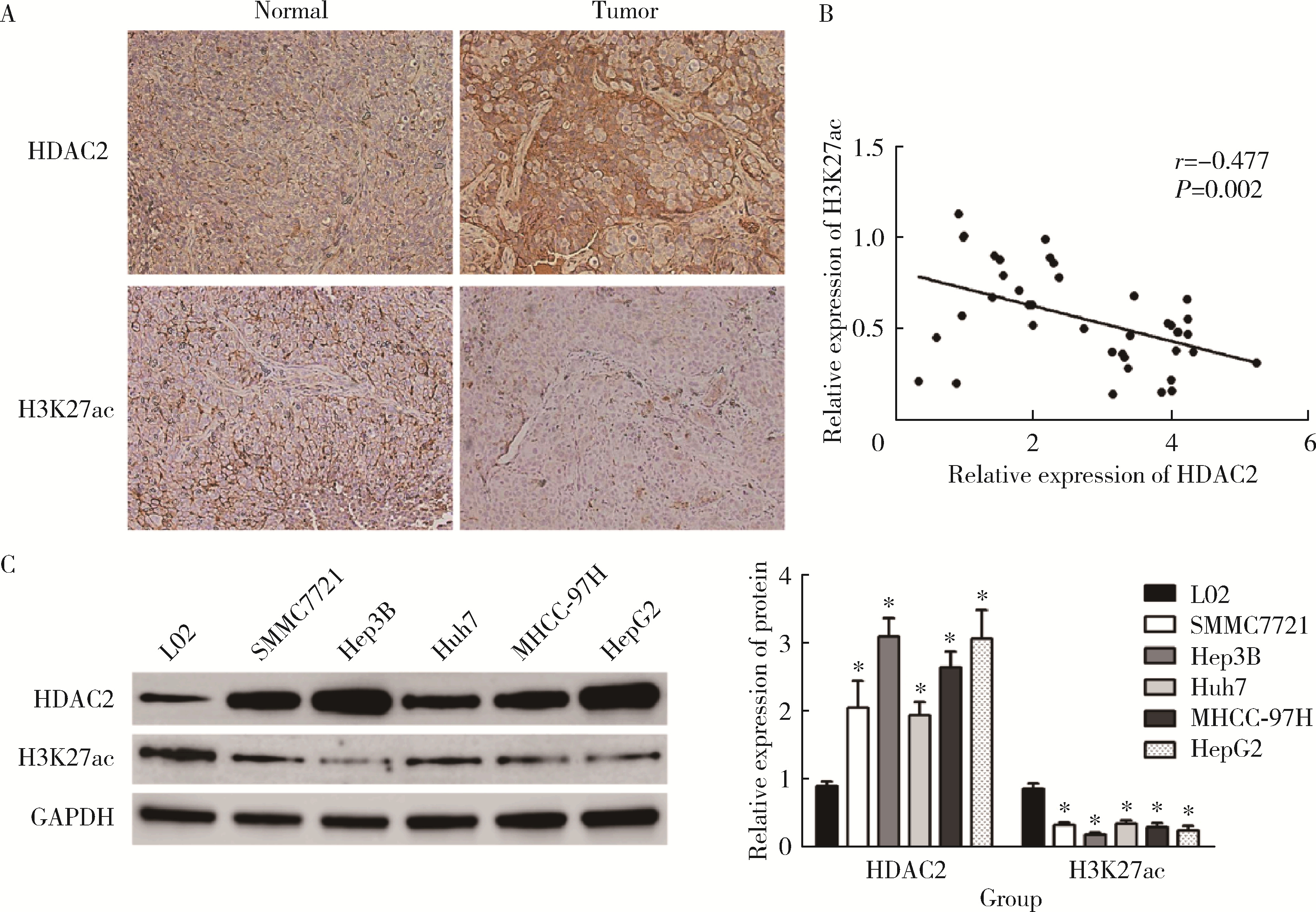
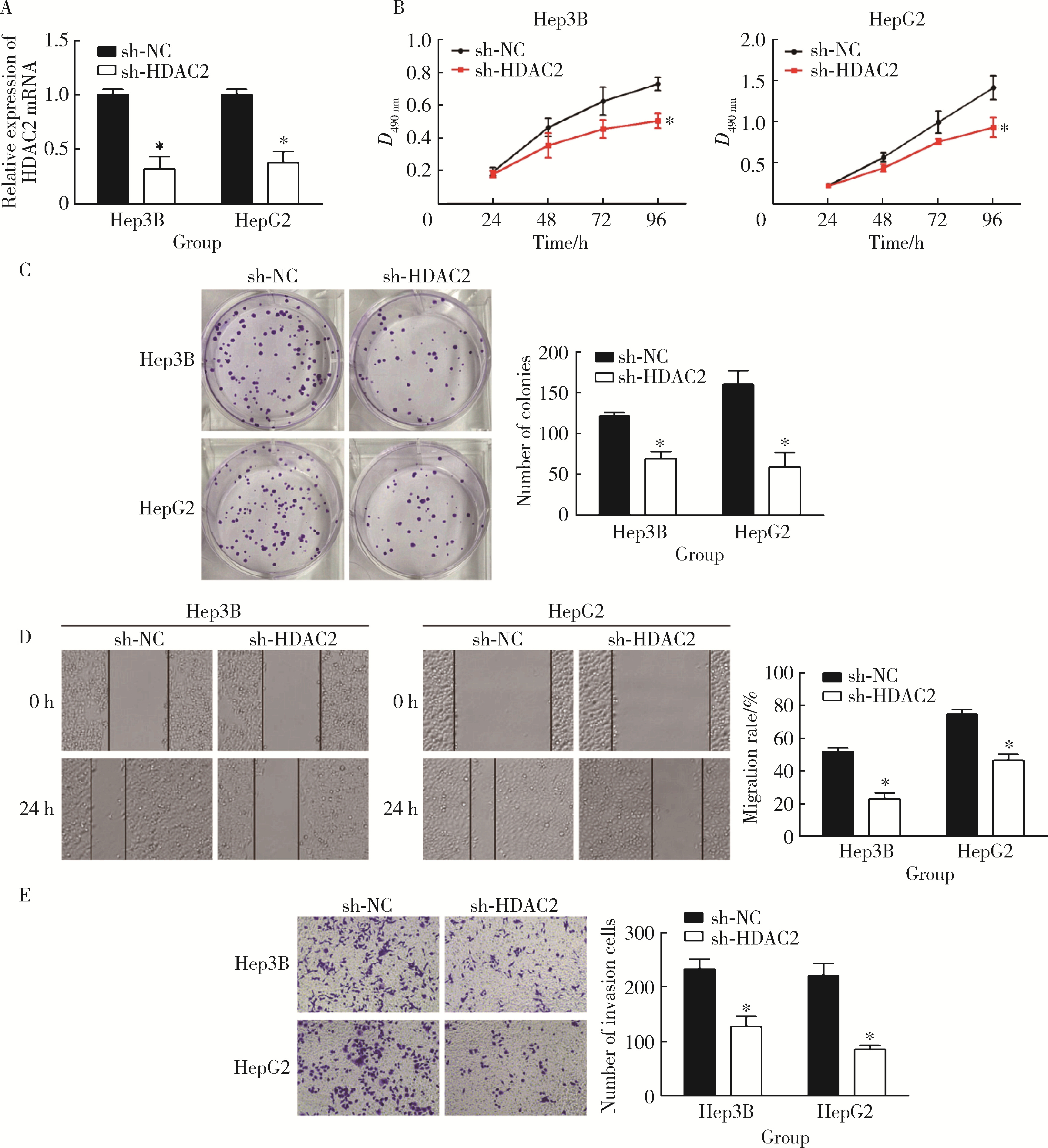
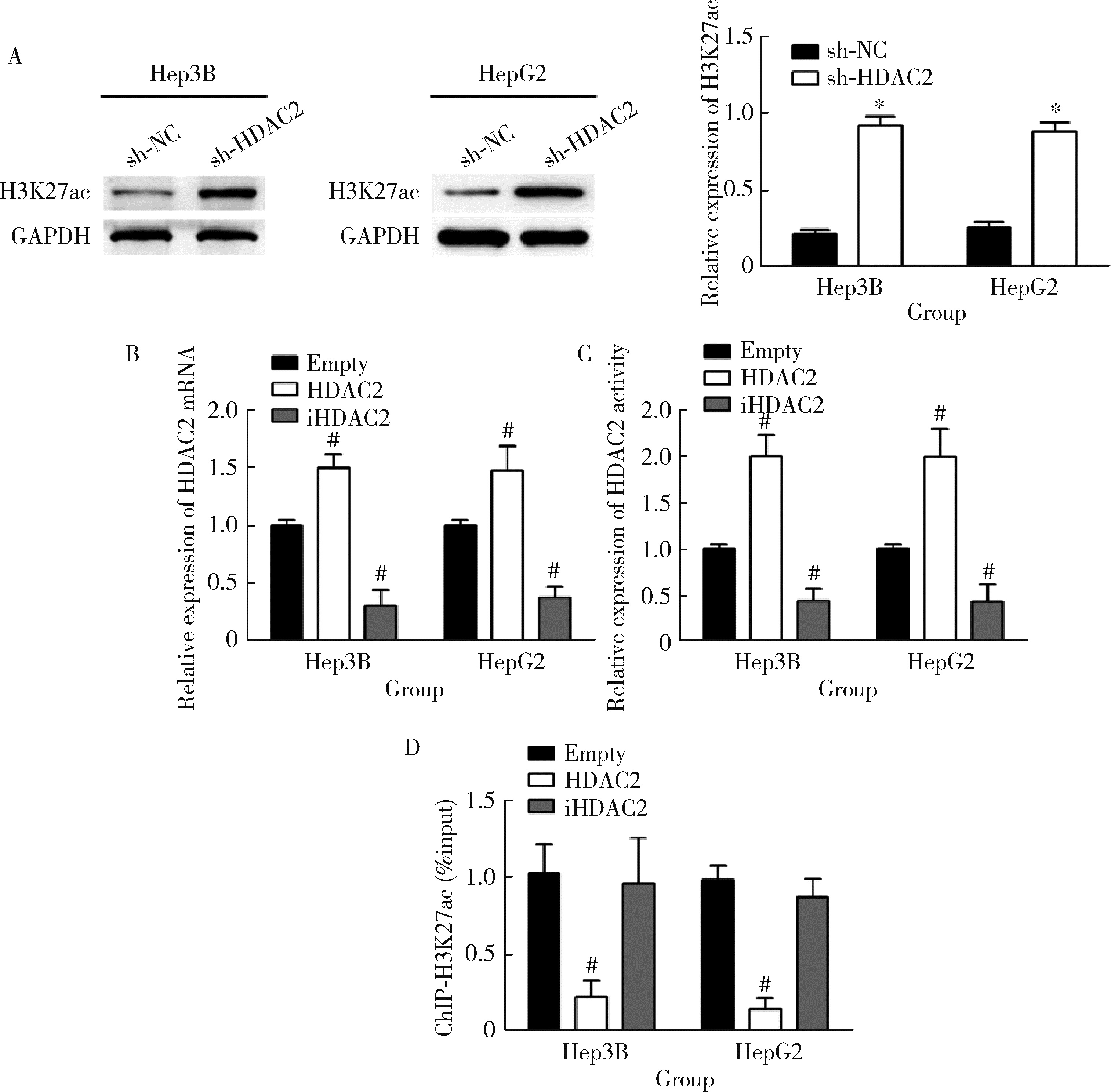
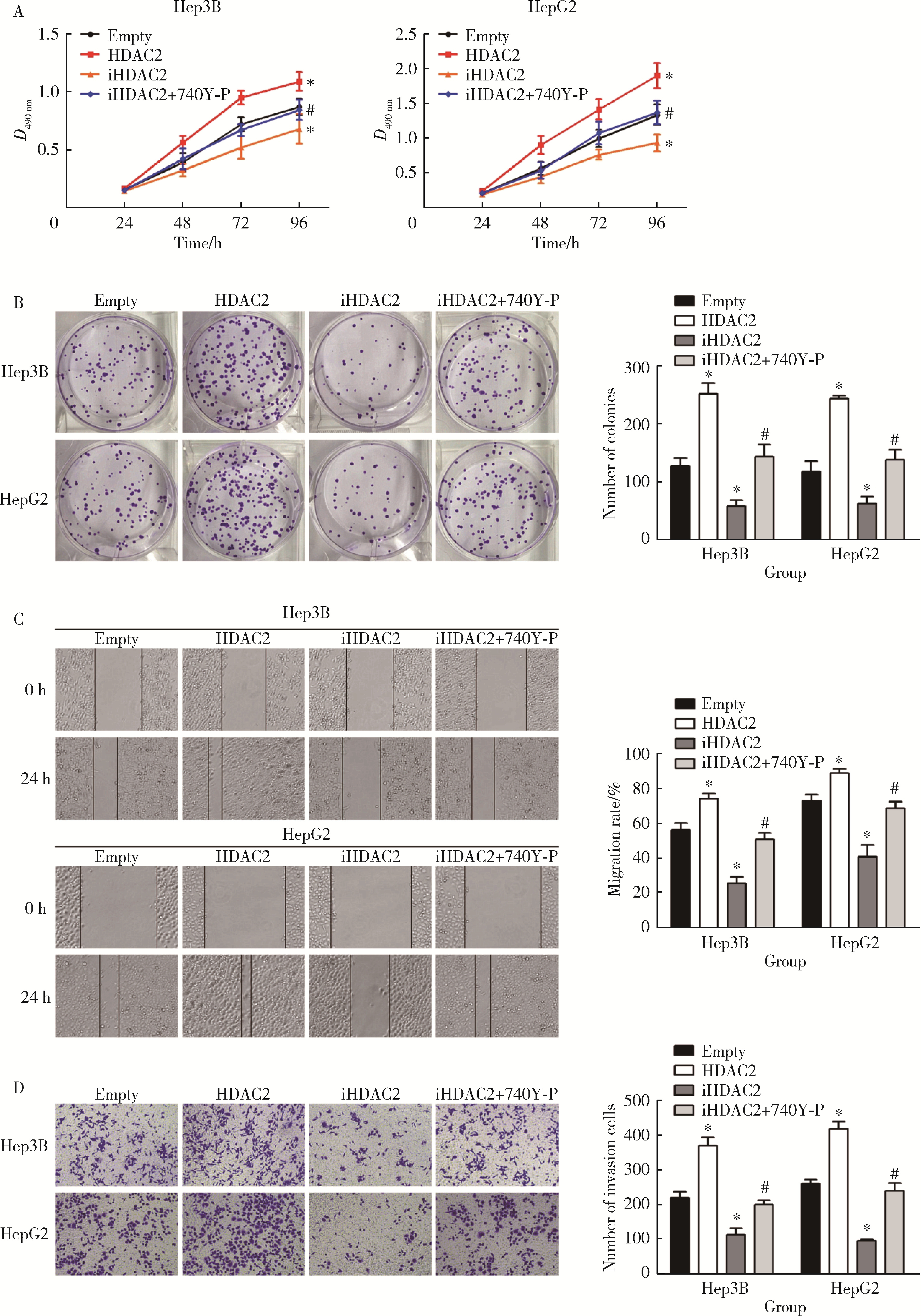
HDAC2-mediated H3K27 acetylation promotes the proliferation and migration of hepatocellular carcinoma cells
Shaohai TANG1, Baoming YANG1, Jiankun LI1, Lili ZHAO2, Yifan WANG3, Shunxiang WANG1,*( )
)
- 1. Department of Hepatobiliary Surgery, the Fourth Hospital of Hebei Medical University, Shijiazhuang 050000, China
2. College of Pharmacy, Hebei Medical University, Shijiazhuang 050000, China;
3. Department of Colorectal Surgery, the Fourth Hospital of Hebei Medical University, Shijiazhuang 050000, China
CLC Number:
- R735.7
| 1 |
doi: 10.1038/s41568-021-00383-9 |
| 2 |
doi: 10.1016/j.cmet.2022.05.003 |
| 3 |
doi: 10.1080/17474124.2021.1991792 |
| 4 |
doi: 10.1002/hep.32740 |
| 5 |
doi: 10.3748/wjg.v28.i3.310 |
| 6 |
doi: 10.1186/s13046-021-01968-w |
| 7 |
doi: 10.1186/s12885-022-10247-6 |
| 8 |
doi: 10.3390/cancers13061265 |
| 9 |
doi: 10.1021/acs.jpcb.1c00694 |
| 10 |
doi: 10.1186/s13148-021-01126-1 |
| 11 |
doi: 10.1007/s12029-020-00370-7 |
| 12 |
doi: 10.3390/molecules27082568 |
| 13 |
|
| 14 |
|
| 15 |
doi: 10.1002/cmdc.202000643 |
| 16 |
姜健, 王维, 崔羽楠, 等. 基于2018版肝脏影像报告及数据系统评估CT及MRI对小于等于3 cm肝细胞性肝癌的诊断价值[J]. 磁共振成像, 2021, 12(9): 25-29, 44.
|
| 17 |
doi: 10.3390/genes12020208 |
| 18 |
doi: 10.1038/s12276-022-00812-1 |
| 19 |
doi: 10.1016/j.molcel.2021.12.004 |
| 20 |
|
| 21 |
doi: 10.1038/s41580-021-00441-y |
| 22 |
|
| 23 |
doi: 10.1002/tox.23516 |
| 24 |
doi: 10.1038/s41401-021-00765-7 |
| 25 |
doi: 10.3390/cancers14061538 |
| 26 |
doi: 10.1038/s41598-021-93815-3 |
| 27 |
doi: 10.1186/s12885-021-08114-x |
| 28 |
doi: 10.1515/biol-2021-0101 |
| 29 |
doi: 10.3892/ijmm.2021.4964 |
| 30 |
doi: 10.1002/tox.23802 |
| 31 |
doi: 10.1038/s41420-021-00750-3 |
| [1] | Yao ZHANG,Jinxin GUO,Shijia ZHAN,Enyu HONG,Hui YANG,Anna JIA,Yan CHANG,Yongli GUO,Xuan ZHANG. Role and mechanism of cysteine and glycine-rich protein 2 in the malignant progression of neuroblastoma [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 495-504. |
| [2] | Lei WANG,Tian-dong HAN,Wei-xing JIANG,Jun LI,Dao-xin ZHANG,Ye TIAN. Comparison of safety and effectiveness of active migration technique and in situ lithotripsy technique in the treatment of 1-2 cm upper ureteral calculi by flexible ure-teroscopy [J]. Journal of Peking University (Health Sciences), 2023, 55(3): 553-557. |
| [3] | YANG Duo,ZHOU Xin-na,WANG Shuo,WANG Xiao-li,YUAN Yan-hua,YANG Hua-bin,GENG Hui-zhen,PENG Bing,LI Zi-bo,LI Bin,REN Jun. Assessment of lymphocytic function in vitro stimulated by specific tumor polypeptide combined with dendritic cells [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1094-1098. |
| [4] | PANG Yong,ZHANG Sha,YANG Hua,ZHOU Rou-li. Serum LAPTM4B-35 protein as a novel diagnostic marker for hepatocellular carcinoma [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 710-715. |
| [5] | Bo MA,Zhi-hua TIAN,Li QU,Yue-xiang LIU,Hong ZHANG,Hui-rong DING. Establishment and gene expression analysis of drug-resistant cell lines in hepatocellular carcinoma induced by sorafenib [J]. Journal of Peking University (Health Sciences), 2020, 52(2): 207-213. |
| [6] | Xin-yun YAO,Xiao-min GAO,Xiao-ying ZOU,Lin YUE. Role of endocytosis in cell surface CXC chemokine receptor 4 expression of stem cells from apical papilla [J]. Journal of Peking University(Health Sciences), 2019, 51(5): 893-899. |
| [7] | Jing XIE,Yu-ming ZHAO,Nan-quan RAO,Xiao-tong WANG,Teng-jiao-zi FANG,Xiao-xia LI,Yue ZHAI,Jing-zhi LI,Li-hong GE,Yuan-yuan WANG. Comparative study of differentiation potential of mesenchymal stem cells derived from orofacial system into vascular endothelial cells [J]. Journal of Peking University(Health Sciences), 2019, 51(5): 900-906. |
| [8] | Jing ZHANG,Jie CHEN,Gui-wen GUAN,Ting ZHANG,Feng-min LU,Xiang-mei CHEN. Expression and clinical significance of chemokine CXCL10 and its receptor CXCR3 in hepatocellular carcinoma [J]. Journal of Peking University(Health Sciences), 2019, 51(3): 402-408. |
| [9] | Yun-bo XIE,Ji-yuan ZHANG,Mei-ling DU,Fan-ping MENG,Jun-liang FU,Li-min LIU,Song-shan WANG,Rui QU,Fang LIAN,Fei QIAO,Yang-liu CHEN,Ying-ying GAO,Ruo-nan XU,Ming SHI,Fu-sheng WANG. Efficacy and peripheral immunity analysis of allogeneic natural killer cells therapy in patients with hepatocellular carcinoma [J]. Journal of Peking University(Health Sciences), 2019, 51(3): 591-595. |
| [10] | Fan ZHANG,Tai-qiang YAN,Wei GUO. Rasfonin inhibits proliferation and migration of osteosarcoma 143B cells [J]. Journal of Peking University(Health Sciences), 2019, 51(2): 234-238. |
| [11] | WANG Zi-cheng, CHENG Li, LV Tong-de, SU Li, LIN Jian, ZHOU Li-qun. Inflammatory priming adipose derived stem cells significantly inhibit the proliferation of peripheral blood mononuclear cells [J]. Journal of Peking University(Health Sciences), 2018, 50(4): 590-594. |
| [12] | TANG Xu, ZHAO Wei-hong, SONG Qin-qin, YIN Hua-qi, DU Yi-qing, SHENG Zheng-zuo, WANG Qiang, ZHANG Xiao-wei, LI Qing, LIU Shi-jun, XU Tao. Influence of SOX10 on the proliferation and invasion of prostate cancer cells [J]. Journal of Peking University(Health Sciences), 2018, 50(4): 602-606. |
| [13] | WANG Xiao-tong, RAO Nan-quan, FANG Teng-jiao-zi, ZHAO Yu-ming, GE Li-hong. Comparison of the properties of CD146 positive and CD146 negative subpopulations of stem cells from human exfoliated deciduous teeth [J]. Journal of Peking University(Health Sciences), 2018, 50(2): 284-292. |
| [14] | CHEN Wei, HU Fan-lei, LIU Hong-jiang, XU Li-ling, LI Ying-ni, LI Zhan-guo. Myeloid-derived suppressor cells promoted autologous B cell proliferation in rheumatoid arthritis [J]. Journal of Peking University(Health Sciences), 2017, 49(5): 819-823. |
| [15] | CAI Yi, GUO Hao, LI Han-zhong, WANG Wen-da, ZHANG Yu-shi. MicroRNA differential expression profile in tuberous sclerosis complex cell line TSC2-/- MEFs and normal cell line TSC2+/+ MEFs [J]. Journal of Peking University(Health Sciences), 2017, 49(4): 580-584. |
|
||