Journal of Peking University (Health Sciences) ›› 2025, Vol. 57 ›› Issue (6): 1051-1060. doi: 10.19723/j.issn.1671-167X.2025.06.006
Previous Articles Next Articles
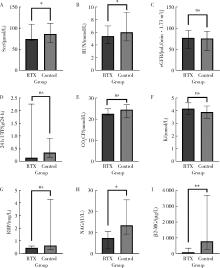
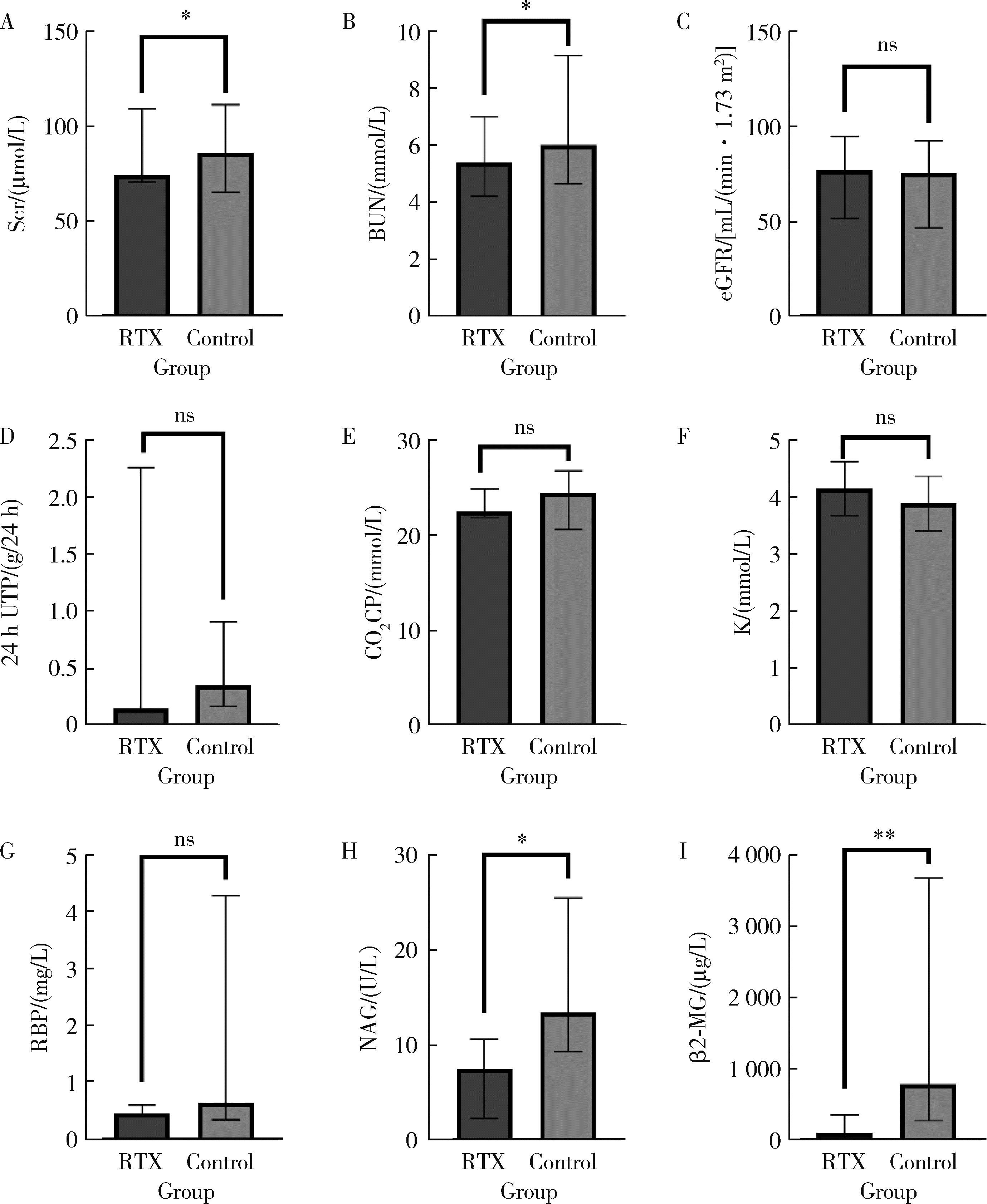
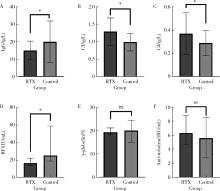
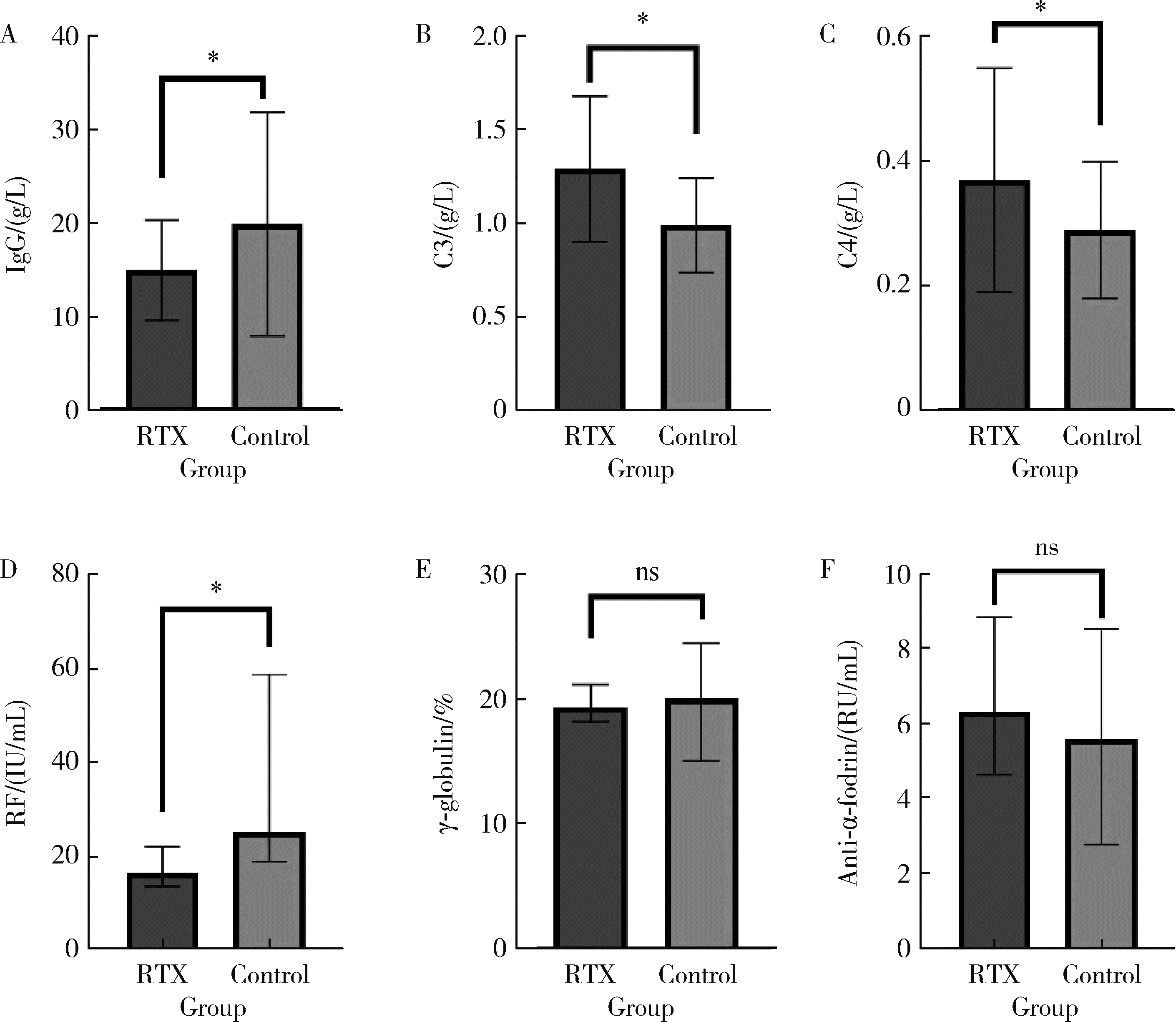
Clinical efficacy and safety of rituximab in treating renal injury in primary Sjögren syndrome
Yayun ZHAO1,2, Mengfan NI3, Xue LI1, Bei WANG1,4, Gong CHENG1, Jing HE1, Yuebo JIN1,*( )
)
- 1. Department of Rheumatology and Immunology, Peking University People' s Hospital, Beijing 100044, China
2. Department of Rheumatology, Hebei Provincial Hospital of Chinese Medicine, Shijiazhuang 050000, China
3. Department of Nephrology, Peking University People' s Hospital, Beijing 100044, China
4. Department of Rheumatology and Immunology, Qiandongnan People' s Hospital, Kaili 556000, Guizhou, China
CLC Number:
- R593.2
| 1 |
doi: 10.1056/NEJMcp1702514 |
| 2 |
doi: 10.2215/CJN.00980209 |
| 3 |
doi: 10.1002/art.38100 |
| 4 |
doi: 10.1136/annrheumdis-2019-216114 |
| 5 |
doi: 10.1186/ar4359 |
| 6 |
doi: 10.1038/s41584-024-01135-3 |
| 7 |
|
| 8 |
|
| 9 |
北京大学医学部肾脏病学系专家组. 利妥昔单抗在膜性肾病中应用的专家共识[J]. 中华内科杂志, 2022, 61(3): 282- 290.
|
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
doi: 10.1038/s41573-020-00092-2 |
| 14 |
doi: 10.1172/JCI40231 |
| 15 |
doi: 10.1002/art.22536 |
| 16 |
doi: 10.1136/annrheumdis-2011-200086 |
| 17 |
doi: 10.7326/M13-1085 |
| 18 |
doi: 10.1007/s10067-005-0086-0 |
| 19 |
doi: 10.1177/0961203314546023 |
| 20 |
中国初级卫生保健基金会风湿免疫学专业委员会. 干燥综合征超药品说明书用药中国临床实践指南(2023版)[J]. 中华医学杂志, 2023, 103(43): 3445- 3461.
|
| 21 |
中国初级卫生保健基金会风湿免疫学分会干燥综合征和IgG4相关性疾病专委会, 系统性红斑狼疮专委会. 利妥昔单抗治疗风湿免疫病中国专家共识(2024版)[J]. 中华风湿病学杂志, 2024, 28(8): 521- 537.
|
| 22 |
doi: 10.3389/fphar.2021.731122 |
| [1] | Yan DING, Lifang WANG, Chaoran LI, Zhemin LU, Lianjie SHI. Rheumatoid arthritis combined with IgG4-related disease successfully treated with rituximab: A case report [J]. Journal of Peking University (Health Sciences), 2025, 57(6): 1203-1207. |
| [2] | Zhao XIANG, Li YANG, Jing YANG. Untargeted metabolomics reveals differential serum metabolites and metabolic pathways in patients with primary Sjögren syndrome and thrombocytopenia [J]. Journal of Peking University (Health Sciences), 2025, 57(6): 1042-1050. |
| [3] | Wenhao LIN, Yang XIE, Fangqing WANG, Shuying WANG, Xiangjun LIU, Fanlei HU, Yuan JIA. Single-cell RNA sequencing of B cells reveals molecular typing in Sjögren syndrome [J]. Journal of Peking University (Health Sciences), 2025, 57(6): 1032-1041. |
| [4] | Yuan LIU, Guixiu SHI. Change from Sjögren syndrome to Sjögren disease [J]. Journal of Peking University (Health Sciences), 2025, 57(6): 1015-1017. |
| [5] | Lixiu ZHU, Renli CHEN, Sujuan ZHOU, Ye LIN, Yirong TANG, Zhen YE. Effect of aquaporin 5 on TLR4/MyD88/NF-κB signaling pathway in Sjögren syndrome rats [J]. Journal of Peking University (Health Sciences), 2025, 57(5): 875-883. |
| [6] | Doudou MA, Zhemin LU, Qian GUO, Sha ZHU, Jin GU, Yan DING, Lianjie SHI. Successful treatment of rheumatoid arthritis complicated with myasthenia gravis with low-dose rituximab: A case report [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 1110-1114. |
| [7] | Jie WU,Wen ZHANG,Shu LIANG,Yi-lu QIN,Wen-qiang FAN. Pregnancy-associated neuromyelitis optical spectrum disorder combined with primary Sjögren's syndrome: A critical illness case report [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1118-1124. |
| [8] | Cai-peng QIN,Fei WANG,Yi-qing DU,Xiao-wei ZHANG,Qing LI,Shi-jun LIU,Tao XU. Diagnosis and treatment of four cases of asymptomatic and non-hydrous ureteral calculi [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 939-942. |
| [9] | YI Wen-xia,WEI Cui-jie,WU Ye,BAO Xin-hua,XIONG Hui,CHANG Xing-zhi. Long-term rituximab treatment of refractory idiopathic inflammatory myopathy: A report of 3 cases [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1191-1195. |
| [10] | Qiu-yu LI,Qin CHENG,Zhi-ling ZHAO,Ni-ni DAI,Lin ZENG,Lan ZHU,Wei GUO,Chao LI,Jun-hong WANG,Shu LI,Qing-gang GE,Ning SHEN. Severe acute respiratory syndrome coronavirus 2 infection in renal transplant recipients: A case report [J]. Journal of Peking University (Health Sciences), 2020, 52(4): 780-784. |
|
||