Journal of Peking University (Health Sciences) ›› 2025, Vol. 57 ›› Issue (6): 1042-1050. doi: 10.19723/j.issn.1671-167X.2025.06.005
Previous Articles Next Articles
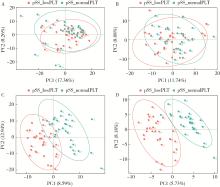
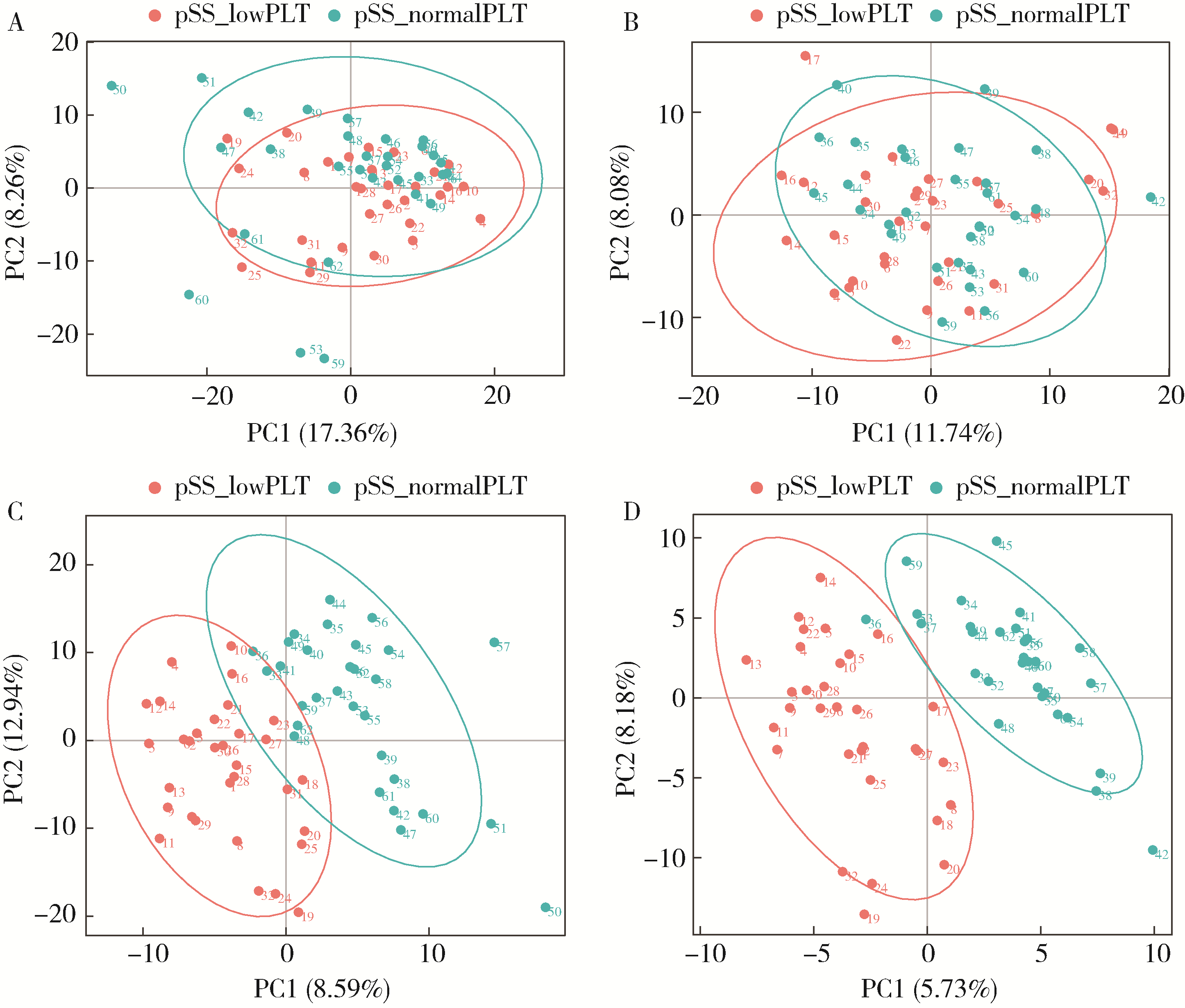
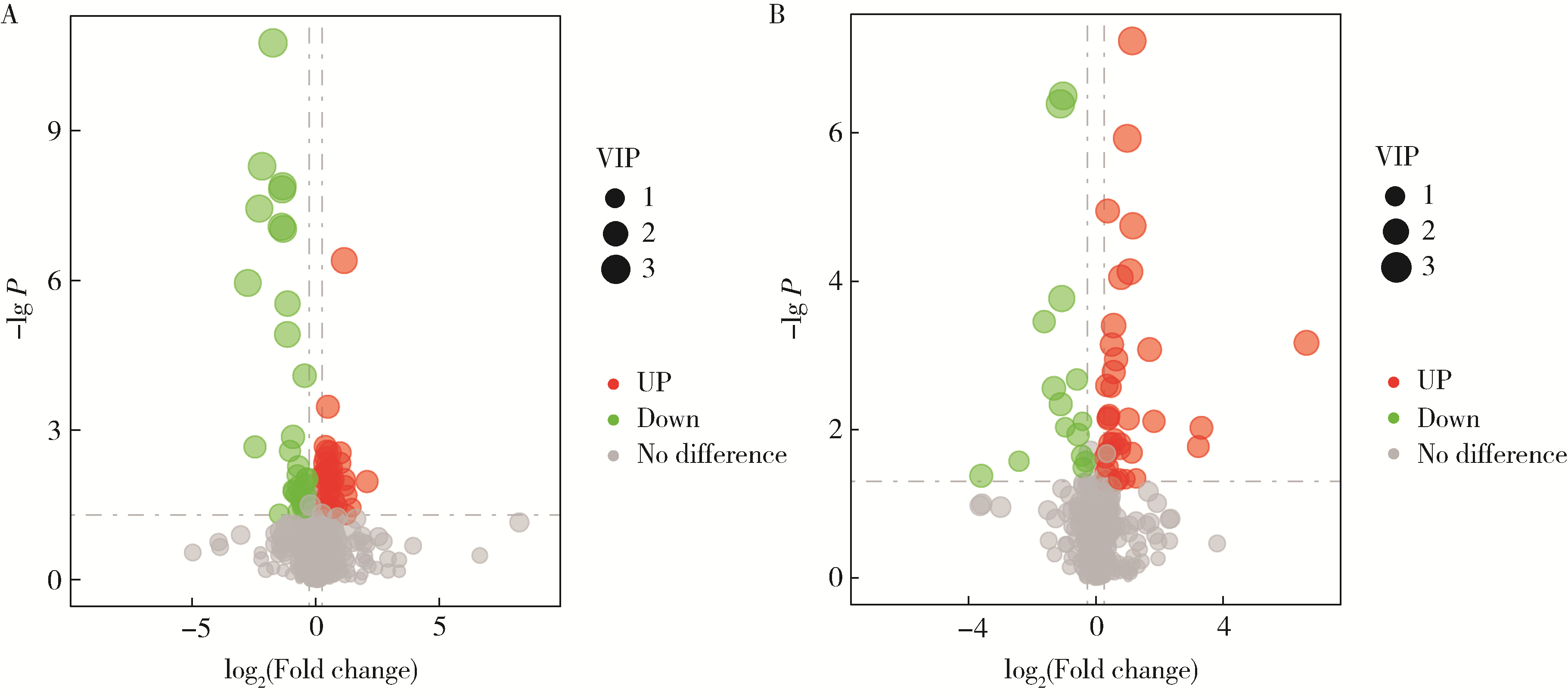
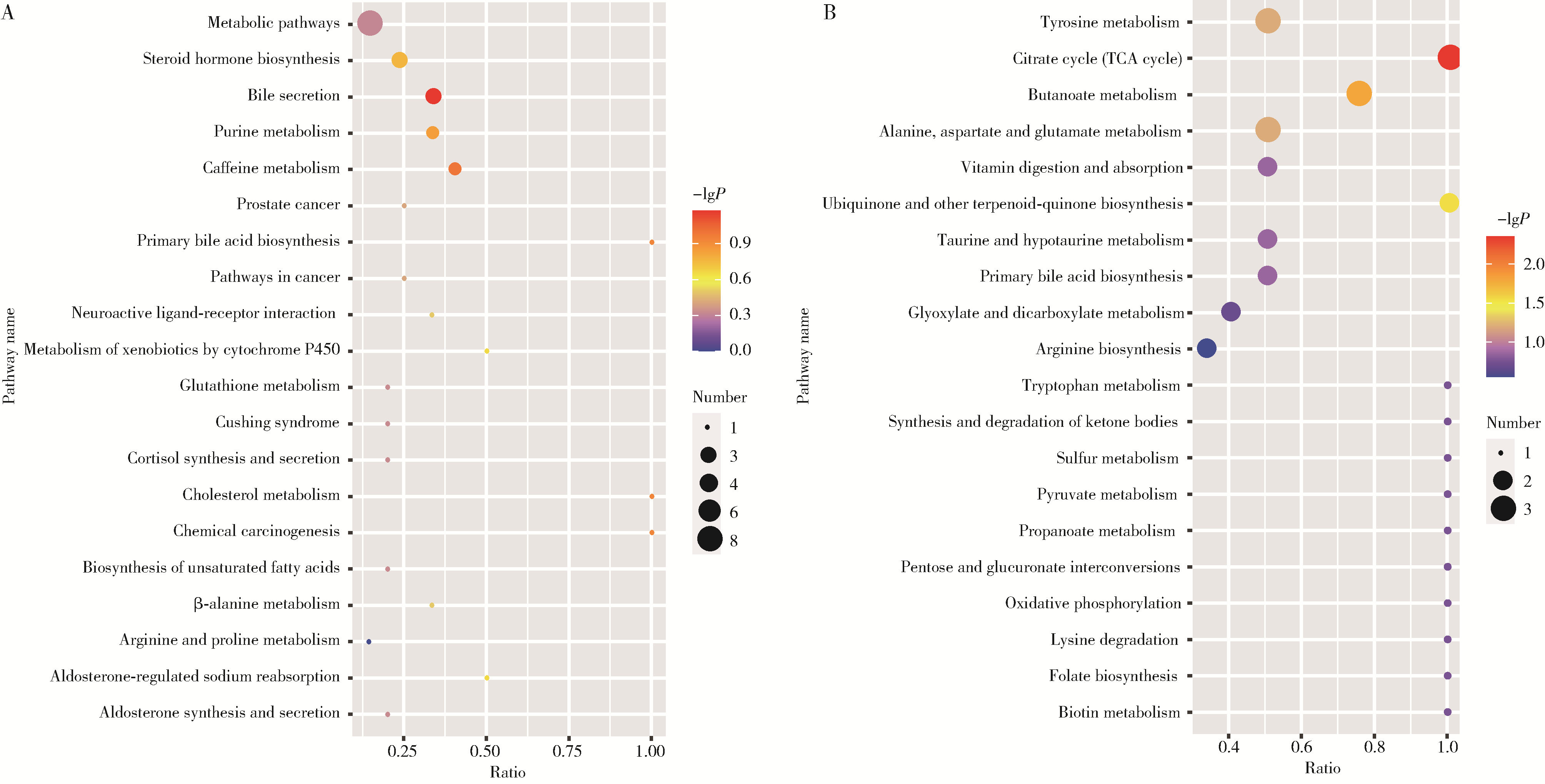
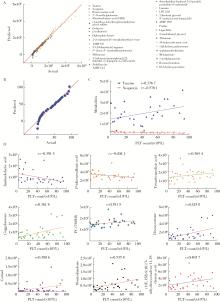
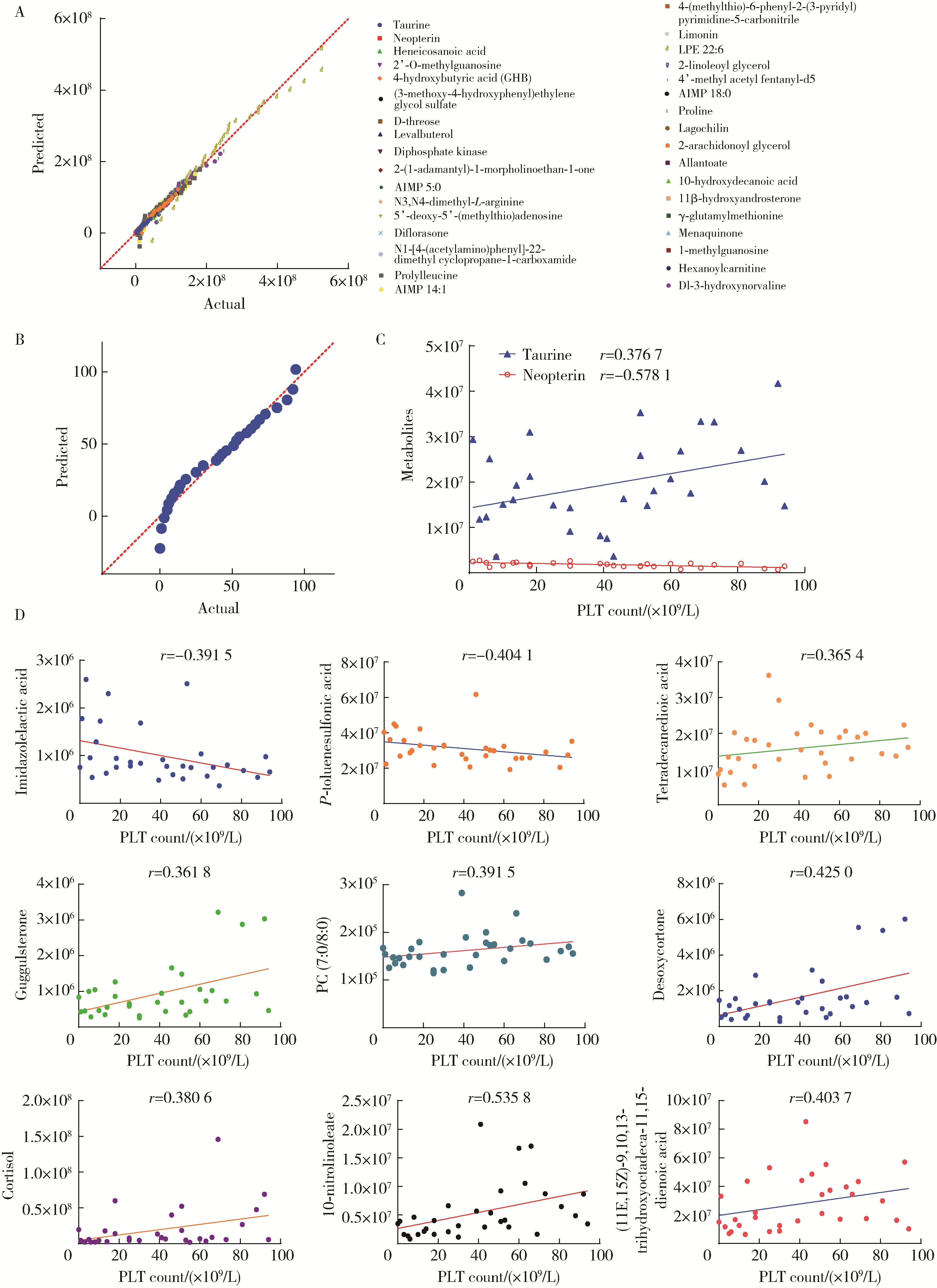
Untargeted metabolomics reveals differential serum metabolites and metabolic pathways in patients with primary Sjögren syndrome and thrombocytopenia
Zhao XIANG1, Li YANG2, Jing YANG2,*( )
)
- 1. Department of Rheumatology and Immunology, Dazhou Central Hospital, Dazhou 625000, Sichuan, China
2. Department of Rheumatology and Immunology, Mianyang Central Hospital, Mianyang 621000, Sichuan, China
CLC Number:
- R593.2
| 1 |
doi: 10.1038/s41467-021-23472-7 |
| 2 |
doi: 10.1056/NEJMcp1702514 |
| 3 |
|
| 4 |
doi: 10.1002/acr.21610 |
| 5 |
王英, 蓝晶莹, 石桂秀. 原发性干燥综合征重度血小板减少患者的临床及免疫学特点分析[J]. 内科急危重症杂志, 2022, 28(2): 103- 107.
|
| 6 |
秦伟, 李四强. 自身免疫性疾病介导的血小板减少机制研究进展[J]. 中国基层医药, 2019, 26(23): 2941- 2944.
|
| 7 |
doi: 10.1002/rth2.12691 |
| 8 |
|
| 9 |
doi: 10.1177/0961203314547796 |
| 10 |
薛媛, 徐东, 李梦涛, 等. 原发性干燥综合征合并严重血小板减少症患者的治疗反应预测[J]. 中华内科杂志, 2019, 58(4): 282- 287.
|
| 11 |
doi: 10.1136/annrheumdis-2019-216114 |
| 12 |
doi: 10.1007/s00296-010-1395-4 |
| 13 |
doi: 10.1080/09537104.2019.1678121 |
| 14 |
|
| 15 |
doi: 10.1038/nbt.4101 |
| 16 |
doi: 10.1016/j.jhep.2019.11.009 |
| 17 |
doi: 10.1093/rheumatology/keaa456 |
| 18 |
doi: 10.3390/ijms22168997 |
| 19 |
doi: 10.1021/acs.jproteome.0c00179 |
| 20 |
张紫妍. 基于GC-MS的ITP患者的血浆代谢组学研究[D]. 苏州: 苏州大学, 2021.
|
| 21 |
doi: 10.3390/ijms23147978 |
| 22 |
doi: 10.1002/art.39859 |
| 23 |
doi: 10.1080/17512433.2021.1903315 |
| 24 |
doi: 10.1038/srep31424 |
| 25 |
doi: 10.1038/ncomms5753 |
| 26 |
doi: 10.1038/nature07762 |
| 27 |
doi: 10.1371/journal.pone.0159384 |
| 28 |
doi: 10.1007/s10067-018-4021-6 |
| 29 |
|
| 30 |
doi: 10.1101/gad.1128003 |
| 31 |
|
| 32 |
|
| 33 |
doi: 10.1111/j.1476-5381.1974.tb09707.x |
| 34 |
doi: 10.1016/j.bbrc.2017.06.079 |
| 35 |
doi: 10.1016/j.pathophys.2018.04.001 |
| 36 |
doi: 10.3389/fimmu.2020.586527 |
| 37 |
doi: 10.1053/j.sult.2013.12.001 |
| 38 |
|
| [1] | Yuan LIU, Guixiu SHI. Change from Sjögren syndrome to Sjögren disease [J]. Journal of Peking University (Health Sciences), 2025, 57(6): 1015-1017. |
| [2] | Wenhao LIN, Yang XIE, Fangqing WANG, Shuying WANG, Xiangjun LIU, Fanlei HU, Yuan JIA. Single-cell RNA sequencing of B cells reveals molecular typing in Sjögren syndrome [J]. Journal of Peking University (Health Sciences), 2025, 57(6): 1032-1041. |
| [3] | Yayun ZHAO, Mengfan NI, Xue LI, Bei WANG, Gong CHENG, Jing HE, Yuebo JIN. Clinical efficacy and safety of rituximab in treating renal injury in primary Sjögren syndrome [J]. Journal of Peking University (Health Sciences), 2025, 57(6): 1051-1060. |
| [4] | Xiangyu SUN, Chao YUAN, Xinzhu ZHOU, Jing DIAO, Shuguo ZHENG. Application of salivary micro-ecosystem in early prevention and control of oral and systemic diseases [J]. Journal of Peking University (Health Sciences), 2025, 57(5): 859-863. |
| [5] | Lixiu ZHU, Renli CHEN, Sujuan ZHOU, Ye LIN, Yirong TANG, Zhen YE. Effect of aquaporin 5 on TLR4/MyD88/NF-κB signaling pathway in Sjögren syndrome rats [J]. Journal of Peking University (Health Sciences), 2025, 57(5): 875-883. |
| [6] | Zhenying BAO, Yajie WANG. Application of combined detection of inflammatory indexes and cytokines in chronic periodontitis [J]. Journal of Peking University (Health Sciences), 2025, 57(4): 772-778. |
| [7] | Zhong CAO,Hong-bing CEN,Jian-hong ZHAO,Jun MEI,Ling-zhi QIN,Wei LIAO,Qi-lin AO. Expression and significance of INSM1 and SOX11 in pancreatic neuroendocrine tumor and solid pseudopapillary neoplasm [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 575-581. |
| [8] | Li LIANG,Xin LI,Lin NONG,Ying DONG,Ji-xin ZHANG,Dong LI,Ting LI. Analysis of microsatellite instability in endometrial cancer: The significance of minimal microsatellite shift [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 254-261. |
| [9] | Fang CAO,Ming ZHONG,Cong-rong LIU. Uterine POLE mutant endometrioid carcinoma combined with human papilloma virus-associated cervical adenocarcinoma: A case report and literature review [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 370-374. |
| [10] | Xue-ping WANG,Yu-ya-nan ZHANG,Tian-lan LU,Zhe LU,Zhe-wei KANG,Yao-yao SUN,Wei-hua YUE. Variations in fecal microbiota of first episode schizophrenia associated with clinical assessment and serum metabolomics [J]. Journal of Peking University (Health Sciences), 2022, 54(5): 863-873. |
| [11] | Yuan MA,Yue ZHANG,Rui LI,Shu-wei DENG,Qiu-shi QIN,Liu-luan ZHU. Characteristics of amino acid metabolism in myeloid-derived suppressor cells in septic mice [J]. Journal of Peking University (Health Sciences), 2022, 54(3): 532-540. |
| [12] | PANG Yong,ZHANG Sha,YANG Hua,ZHOU Rou-li. Serum LAPTM4B-35 protein as a novel diagnostic marker for hepatocellular carcinoma [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 710-715. |
| [13] | ZHOU Chuan, MA Xue, XING Yun-kun, LI Lu-di, CHEN Jie, YAO Bi-yun, FU Juan-ling, ZHAO Peng. Exploratory screening of potential pan-cancer biomarkers based on The Cancer Genome Atlas database [J]. Journal of Peking University (Health Sciences), 2021, 53(3): 602-607. |
| [14] | CHEN Huai-an,LIU Shuo,LI Xiu-jun,WANG Zhe,ZHANG Chao,LI Feng-qi,MIAO Wen-long. Clinical value of inflammatory biomarkers in predicting prognosis of patients with ureteral urothelial carcinoma [J]. Journal of Peking University (Health Sciences), 2021, 53(2): 302-307. |
| [15] | Qian GUO, Xiao-xu MA, Hui GAO, Lian-jie SHI, Yu-chao ZHONG, Lin-feng XIE, Miao SHAO, Xue-wu ZHANG. Association of Semaphorin 3A with thrombocytopenia in systemic lupus erythematosus [J]. Journal of Peking University (Health Sciences), 2020, 52(5): 892-896. |
|
||