Journal of Peking University(Health Sciences) ›› 2020, Vol. 52 ›› Issue (1): 24-29. doi: 10.19723/j.issn.1671-167X.2020.01.004
Previous Articles Next Articles
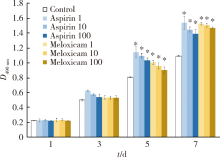
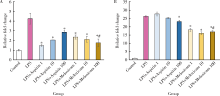
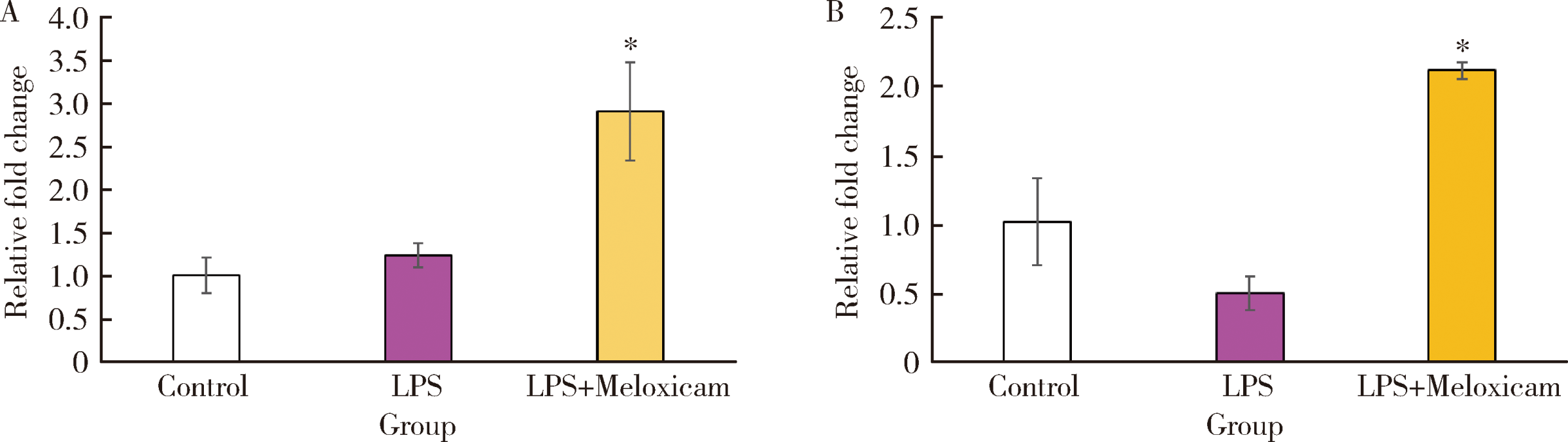
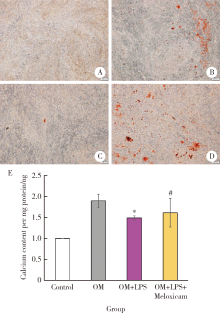
Anti-inflammatory and repaired effects of non-steroidal anti-inflammatory drugs on human dental pulp cells
Jing-yi LI,Sai-nan WANG,Yan-mei DONG( )
)
- Department of Cariology and Endodoontology, Peking University School and Hospital of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
CLC Number:
- R781.31
| [1] | 董艳梅 . 活髓保存治疗与生物活性盖髓剂的临床现状与研究[J]. 中华口腔医学杂志, 2014,49(5):268-271. |
| [2] | Farges JC, Alliotlicht B, Renard E , et al. Dental pulp defence and repair mechanisms in dental caries [J/OL]. Mediators Inflamm, 2015 ( 2015-10-11). doi: 10.1155/2015/230251. |
| [3] | Farges JC, Carrouel F, Keller JF , et al. Cytokine production by human odontoblast-like cells upon Toll-like receptor-2 engagement[J]. Immunobiology, 2011,216(4):513-517. |
| [4] | Zanini M, Meyer E, Simon S . Pulp inflammation diagnosis from clinical to inflammatory mediators: A systematic review[J]. J Endod, 2017,43(7):1033-1051. |
| [5] | Chang M, Lin L, Zwei-Ching Chang J , et al. Regulation of vascular cell adhesion molecule-1 in dental pulp cells by interleukin-1β: The role of prostanoids[J]. J Endod, 2012,38(6):774-779. |
| [6] | Langeland K . Tissue response to dental caries[J]. Dent Traumatol, 2010,3(4):149-171. |
| [7] | de Waal Malefyt R, Abrams J, Bennett B , et al. Interleukin 10 (IL-10) inhibits cytokine synjournal by human monocytes: An autoregulatory role of IL-10 produced by monocytes[J]. J Exp Med, 1991,174(5):1209-1220. |
| [8] | Feng G, Zheng K, Cao T , et al. Repeated stimulation by LPS promotes the senescence of DPSCs via TLR4/MyD88-NF-κB-p53/p21 signaling[J]. Cytotechnology, 2018,70(3):1023-1035. |
| [9] | Nakane A, Yoshida T, Nakata K , et al. Effects of lipopolysaccharides on human dental pulp cells[J]. J Endod, 1995,21(3):128-130. |
| [10] | Bletsa A, Berggreen E, Fristad I , et al. Cytokine signalling in rat pulp interstitial fluid and transcapillary fluid exchange during lipopolysaccharide-induced acute inflammation[J]. J Physiol, 2006,573(Pt 1):225-236. |
| [11] | Ko YJ, Kwon KY, Kum KY , et al. The anti-inflammatory effect of human telomerase-derived peptide on P. gingivalis lipopolysaccharide-induced inflammatory cytokine production and its mechanism in human dental pulp cells[J]. Mediators Inflamm, 2015 ( 2015-10-28). doi: 10.1155/2015/385127. |
| [12] | Takimoto K, Kawashima N, Suzuki N , et al. Down-regulation of inflammatory mediator synjournal and infiltration of inflammatory cells by MMP-3 in experimentally induced rat pulpitis[J]. J Endod, 2014,40(9):1404-1409. |
| [13] | 王艳丽, 潘克清, 孙艳 , 等. 脂多糖对大鼠牙髓细胞ALP、BSP、DSPP表达的影响[J]. 上海口腔医学, 2014,23(4):431-435. |
| [14] | Warner TD, Giuliano F, Vojnovic I , et al. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: A full in vitro ana-lysis[J]. Proc Natl Acad Sci USA, 1999,96(13):7563-7568. |
| [15] | 史旭波, 胡大一 . 阿司匹林的作用机制及相关临床问题[J]. 临床荟萃, 2008,23(16):1141-1143. |
| [16] | 傅得兴, 封宇飞 . 非甾体类抗炎药的安全性研究[J]. 临床合理用药杂志, 2011,11(4):51-53. |
| [17] | 王平, 顾振纶 . 新型非甾体抗炎药——美洛昔康[J]. 中国新药与临床杂志, 2000,19(6):499-501. |
| [18] | Gronthos S, Mankani M, Brahim J , et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo[J]. Proc Natl Acad Sci USA, 2000,97(25):13625-13630. |
| [19] | 孙蕾, 范晓敏, 何文喜 , 等. 阿司匹林对人牙髓干细胞体外增殖、分化的影响及分子机制的研究[J]. 牙体牙髓牙周病学杂志, 2016,26(4):213-217. |
| [20] | Choi EK, Kim SH, Kang IC , et al. Ketoprofen inhibits expression of inflammatory mediators in human dental pulp cells[J]. J Endod, 2013,39(6):764-767. |
| [21] | Hadjicharalambous C, Alexaki VI, Alpantaki K , et al. Effects of NSAIDs on the osteogenic differentiation of human adipose tissue-derived stromal cells[J]. J Pharm Pharmacol, 2016,68(11):1403-1408. |
| [22] | Müller M, Raabe O, Addicks K , et al. Effects of non-steroidal anti-inflammatory drugs on proliferation, differentiation and migration in equine mesenchymal stem cells[J]. Cell Biol Int, 2010,35(3):235-248. |
| [23] | He W, Wang Z, Luo Z , et al. LPS promote the odontoblastic differentiation of human dental pulp stem cells via MAPK signaling pathway[J]. J Cell Physiol, 2015,230(3):554-561. |
| [24] | Barkhordar RA, Hayashi C, Hussain MZ . Detection of interleukin-6 in human dental pulp and periapical lesions[J]. Endod Dent Traumatol, 1999,15(1):26-27. |
| [25] | Pezelj-Ribaric S, Anic I, Brekalo I , et al. Detection of tumor necrosis factor alpha in normal and inflamed human dental pulps[J]. Arch Med Res, 2002,33(5):482-484. |
| [26] | Lin PS, Cheng RH, Chang MC , et al. TGF-β1 stimulates cyclooxygenase-2 expression and PGE2 production of human dental pulp cells: Role of ALK5/Smad2 and MEK/ERK signal transduction pathways[J]. J Formos Med Assoc, 2017,116(10):748-754. |
| [27] | Nakanishi T, Shimizu H, Hosokawa Y , et al. An immunohistological study on cyclooxygenase-2 in human dental pulp[J]. J Endod, 2001,27(6):385-388. |
| [28] | Chang MC, Hung HP, Lin LD , et al. Effect of interleukin-1β on ICAM-1 expression of dental pulp cells: Role of PI3K/Akt, MEK/ERK, and cyclooxygenase[J]. Clin Oral Investig, 2015,19(1):117-126. |
| [29] | 戈升荣, 王晓珉, 祝德秋 , 等. HPLC法测定美洛昔康血药浓度及其药动学[J]. 中国临床药学杂志, 2004,13(4):231-232. |
| [30] | 申晓靖, 刘海蓉, 刘新燕 , 等. 阿司匹林影响人牙髓间充质干细胞成骨分化的新型药理研究[J]. 中国医学前沿杂志:电子版, 2017,9(1):73-77. |
| [31] | Gurgel BCV, Almeida KT, Peixoto RF , et al. Selective COX-2 inhibitor (meloxicam) and tooth-supporting bone quality. A histomorphometric study in rats[J]. Braz Dent J, 2017,28(2):135-139. |
| [32] | Pablos AB, Ramalho SA, König B Jr , et al. Effect of meloxicam and diclofenac sodium on peri-implant bone healing in rats[J]. J Periodontol, 2008,79(2):300-306. |
| [33] | Kirschneck C, Meier M, Bauer K , et al. Meloxicam medication reduces orthodontically induced dental root resorption and tooth movement velocity: A combined in vivo and in vitro study of dental-periodontal cells and tissue[J]. Cell Tissue Res, 2017,368(1):61-78. |
| [34] | Samoto H, Shimizu E, Matsuda-Honjyo Y , et al. Prostaglandin E2 stimulates bone sialoprotein (BSP) expression through cAMP and fibroblast growth factor 2 response elements in the proximal promoter of the rat BSP gene[J]. J Biol Chem, 2003,278(31):28659-28667. |
| [1] | Jiayi TIAN, Yixue GUO, Xia ZHANG, Xiaolin SUN, Jing HE. Flow cytometry analysis of normal range of natural killer cells and their subsets in peripheral blood of healthy Chinese adults [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 839-844. |
| [2] | Zhihui WU, Mingzhi HU, Qiaoying ZHAO, Fengfeng LV, Jingying ZHANG, Wei ZHANG, Yongfu WANG, Xiaolin SUN, Hui WANG. Immunomodulatory mechanism of umbilical cord mesenchymal stem cells modified by miR-125b-5p in systemic lupus erythematosus [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 860-867. |
| [3] | Yuanzhi JIAN,Fei WANG,Ning YIN,Ruoyu ZHOU,Junbo WANG. Developmental toxicity of Cry1Ab protein in the embryonic stem-cell model [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 213-222. |
| [4] | Yuan PAN,Hang GU,Han XIAO,Lijun ZHAO,Yiman TANG,Wenshu GE. Ubiquitin-specific protease 42 regulates osteogenic differentiation of human adipose-derived stem cells [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 9-16. |
| [5] | Xiang-ge ZHAO,Jia-qing LIU,Hui-na HUANG,Zhi-min LU,Zi-ran BAI,Xia LI,Jing-jing QI. Interferon-α mediating the functional damage of CD56dimCD57+natural killer cells in peripheral blood of systemic lupus erythematosuss [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 975-981. |
| [6] | Lei WANG,Xiang-shu JIN,Hui-jun DONG,Guo-min OU,Xin-yuan LAI,Hui ZHUANG,Tong LI,Kuan-hui XIANG. Establishment of a reporter system for estimating activation of human hepatic stellate cells based on COL1A1 promoter and enhanced green fluorescent protein [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 876-885. |
| [7] | Yu-yang YE,Lin YUE,Xiao-ying ZOU,Xiao-yan WANG. Characteristics and microRNA expression profile of exosomes derived from odontogenic dental pulp stem cells [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 689-696. |
| [8] | Bo-han NING,Qing-xia ZHANG,Hui YANG,Ying DONG. Endometrioid adenocarcinoma with proliferated stromal cells, hyalinization and cord-like formations: A case report [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 366-369. |
| [9] | Xiu-rui LIANG,Xue-chun SHAN,Jing GUAN,Rui ZHANG,Jing YANG,Yi ZHANG,Jia-qi JIN,Yu-xin ZHANG,Fan XU,Ji-hua FU. Role of hyperglycemia-induced 5-hydroxytryptamine degradation of hepatic stellate cells in hepatic inflammation and fibrosis induced by type 2 diabetes mellitus [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1141-1150. |
| [10] | Chao HAN,Zhu-xing ZHOU,You-rong CHEN,Zi-hui DONG,Jia-kuo YU. Biological characteristics of sheep peripheral blood mesenchymal stem cell [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1151-1157. |
| [11] | GAO Peng,LUO Yan-ping,LI Jun-feng,CHEN lin,MAO Xiao-rong. Effects of hepatitis B virus on Th17, Treg and Th17/Treg ratio in different alanine aminetransferase stages [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 272-277. |
| [12] | SHUAI Ting,LIU Juan,GUO Yan-yan,JIN Chan-yuan. Knockdown of long non-coding RNA MIR4697 host gene inhibits adipogenic differentiation in bone marrow mesenchymal stem cells [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 320-326. |
| [13] | TIAN Jing,QIN Man,CHEN Jie,XIA Bin. Early loss of primary molar and permanent tooth germ caused by the use of devitalizer during primary molar root canal therapy: Two cases report [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 381-385. |
| [14] | YANG Duo,ZHOU Xin-na,WANG Shuo,WANG Xiao-li,YUAN Yan-hua,YANG Hua-bin,GENG Hui-zhen,PENG Bing,LI Zi-bo,LI Bin,REN Jun. Assessment of lymphocytic function in vitro stimulated by specific tumor polypeptide combined with dendritic cells [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1094-1098. |
| [15] | MA Xiang-bo,ZHANG Xue-wu,JIA Ru-lin,GAO Ying,LIU Hong-jiang,LIU Yu-fang,LI Ying-ni. Application of lymphocytes test in peripheral blood of patients with systemic sclerosis during the treatment [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 721-727. |
|
||