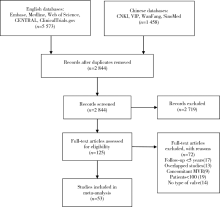
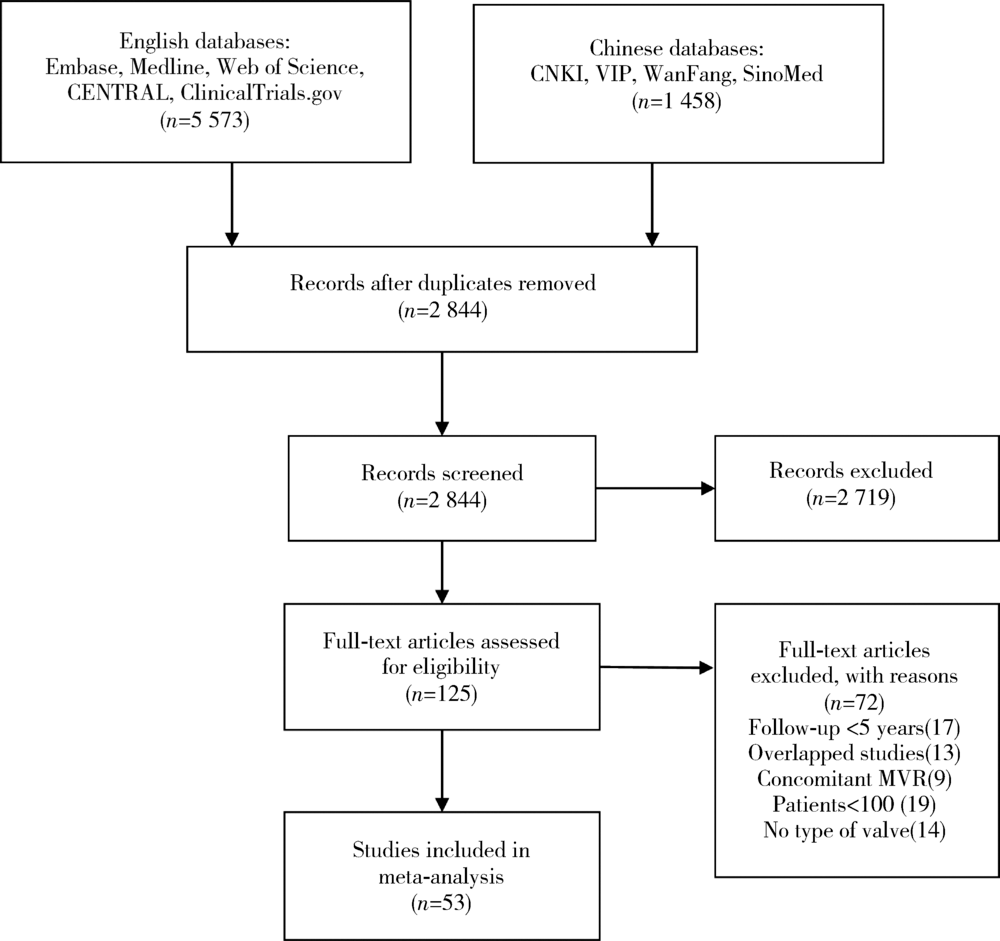
Journal of Peking University (Health Sciences) ›› 2020, Vol. 52 ›› Issue (3): 547-556. doi: 10.19723/j.issn.1671-167X.2020.03.023
Previous Articles Next Articles
Safety of biological valves for aortic valve replacement: A systematic review and meta-analysis
Bao-qi ZENG1,Shu-qing YU1,Yao CHEN1,Wei ZHAI2,Bin LIU2,Si-yan ZHAN1,Feng SUN1,△( )
)
- 1. Department of Epidemiology and Biostatistics, Peking University School of Public Health, Beijing 100191, China
2. Beijing Center for ADR Monitoring, Beijing 100024, China
CLC Number:
- R654.2
| [1] | Ensminger S, Fujita B, Bauer T, et al. Rapid deployment versus conventional bioprosthetic valve replacement for aortic stenosis[J]. J Am Coll Cardiol, 2018,71(13):1417-1428. |
| [2] | Brown JM, O’Brien SM, Wu C, et al. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database[J]. J Thorac Cardiovasc Surg, 2009,137(1):82-90. |
| [3] | Yacoub MH, Takkenberg JJ. Will heart valve tissue engineering change the world?[J]. Nat Clin Pract Cardiovasc Med, 2005,2(2):60-61. |
| [4] | 徐秀林, 张京航, 梁伟, 等. 人工心脏瓣膜质量及安全性评价[J]. 中国医疗器械杂志, 2004,28(5):360-362. |
| [5] | Isaacs AJ, Shuhaiber J, Salemi A, et al. National trends in utilization and in-hospital outcomes of mechanical versus bioprosthetic aortic valve replacements [J]. J Thorac Cardiovasc Surg, 2015, 149(5): 1262-1269.e3. |
| [6] | Dunning J, Gao H, Chambers J, et al. Aortic valve surgery: marked increases in volume and significant decreases in mechanical valve use—an analysis of 41,227 patients over 5 years from the Society for Cardiothoracic Surgery in Great Britain and Ireland National database [J]. J Thorac Cardiovasc Surg, 2011, 142(4): 776-782.e3. |
| [7] |
Siregar S, de Heer F, Groenwold RH, et al. Trends and outcomes of valve surgery: 16-year results of Netherlands Cardiac Surgery National Database[J]. Eur J Cardiothorac Surg, 2014,46(3):386-397.
doi: 10.1093/ejcts/ezu017 pmid: 24554075 |
| [8] | Fujita B, Ensminger S, Bauer T, et al. Trends in practice and outcomes from 2011 to 2015 for surgical aortic valve replacement: an update from the German Aortic Valve Registry on 42776 patients[J]. Eur J Cardiothorac Surg, 2018,53(3):552-559. |
| [9] | Aasbjerg K, Mortensen PE, Norgaard MA, et al. Comparison of survival after aortic valve replacement with mitroflow or perimount prostheses[J]. Semin Thorac Cardiovasc Surg, 2019,31(3):350-358. |
| [10] |
Accola KD, Scott ML, Palmer GJ, et al. Surgical management of aortic valve disease in the elderly: A retrospective comparative study of valve choice using propensity score analysis[J]. J Heart Valve Dis, 2008,17(4):355-365.
pmid: 18751463 |
| [11] |
Albert A, Florath I, Rosendahl U, et al. The late impact of surgical skills and training on the subcoronary implantation of the Freestyle stentless bioprosjournal[J]. J Heart Valve Dis, 2010,19(1):104-114.
pmid: 20329496 |
| [12] | Amabile N, Bical OM, Azmoun A, et al. Long-term results of Freestyle stentless bioprosjournal in the aortic position: a single-center prospective cohort of 500 patients[J]. J Thorac Cardiov Sur, 2014,148(5):1903-1911. |
| [13] |
Andreas M, Wallner S, Ruetzler K, et al. Comparable long-term results for porcine and pericardial prostheses after isolated aortic valve replacement[J]. Eur J Cardiothorac Surg, 2015,48(4):557-561.
doi: 10.1093/ejcts/ezu466 pmid: 25527170 |
| [14] | Anselmi A, Flécher E, Ruggieri VG, et al. Long-term results of the Medtronic Mosaic porcine bioprosjournal in the aortic position[J]. J Thorac Cardiov Sur, 2014,147(6):1884-1891. |
| [15] | Bach DS, Kon ND. Long-term clinical outcomes 15 years after aortic valve replacement with the Freestyle stentless aortic bioprosjournal[J]. Ann Thorac Surg, 2014,97(2):544-551. |
| [16] | Benhameid O, Jamieson WRE, Castella M, et al. CarboMedics mitroflow pericardial aortic bioprosjournal—Performance in patients aged 60 years and older after 15 years[J]. Thorac Cardiov Surg, 2008,56(4):195-199. |
| [17] | Biglioli P, Spampinato N, Cannata A, et al. Long-term outcomes of the Carpentier-Edwards pericardial valve prosjournal in the aortic position: effect of patient age[J]. J Heart Valve Dis, 2004,13(Suppl 1):S49-51. |
| [18] |
Bottio T, Rizzoli G, Thiene G, et al. Hemodynamic and clinical outcomes with the Biocor valve in the aortic position: an 8-year experience[J]. J Thorac Cardiov Sur, 2004,127(6):1616-1623.
doi: 10.1016/j.jtcvs.2003.10.041 |
| [19] |
Bourguignon T, Bouquiaux-Stablo AL, Candolfi P, et al. Very long-term outcomes of the carpentier-edwards perimount valve in aortic position[J]. Ann Thorac Surg, 2015,99(3):831-837.
doi: 10.1016/j.athoracsur.2014.09.030 pmid: 25583467 |
| [20] |
Celiento M, Ravenni G, Tomei L, et al. Excellent durability of the Mosaic porcine aortic bioprosjournal at extended follow up[J]. J Heart Valve Dis, 2018,27(1):97-103.
pmid: 30560605 |
| [21] |
Chan V, Kulik A, Tran A, et al. Long-term clinical and hemodynamic performance of the hancock Ⅱ versus the perimount aortic bioprostheses[J]. Circulation, 2010,122(11 Suppl):S10-16.
doi: 10.1161/CIRCULATIONAHA.109.928085 pmid: 20837899 |
| [22] | Christ T, Grubitzsch H, Claus B, et al. Long-term follow-up after aortic valve replacement with Edwards Prima Plus stentless bioprostheses in patients younger than 60 years of age[J]. J Thorac Cardiov Sur, 2014,147(1):264-269. |
| [23] |
Corbineau H, Verhoye JP, Tauran A, et al. Medtronic intact porcine bioprosjournal in the aortic position: 13-year results[J]. J Heart Valve Dis, 2002,11(4):537-542.
pmid: 12150303 |
| [24] |
David TE, Armstrong S, Maganti M. Hancock Ⅱ bioprosjournal for aortic valve replacement: The gold standard of bioprosthetic valves durability?[J]. Ann Thorac Surg, 2010,90(3):775-781.
doi: 10.1016/j.athoracsur.2010.05.034 |
| [25] |
David TE, Feindel CM, Bos J, et al. Aortic valve replacement with Toronto SPV bioprosjournal: optimal patient survival but suboptimal valve durability[J]. J Thorac Cardiov Sur, 2008,135(1):19-24.
doi: 10.1016/j.jtcvs.2007.04.068 |
| [26] |
de la Fuente A, Sanchez R, Imizcoz A, et al. Intact Medtronic and Carpentier Edwards S.A.V.: clinical and hemodynamic outcomes over 13 years[J]. Cardiovasc Surg, 2003,11(2):139-144.
doi: 10.1016/S0967-2109(03)00010-3 |
| [27] |
Dellgren G, Eriksson MJ, Brodin LÅ, et al. Eleven years’ experience with the Biocor stentless aortic bioprosjournal: Clinical and hemodynamic follow-up with long-term relative survival rate[J]. Eur J Cardiothorac Surg, 2002,22(6):912-921.
doi: 10.1016/s1010-7940(02)00584-5 pmid: 12467813 |
| [28] |
Desai ND, Merin O, Cohen GN, et al. Long-term results of aortic valve replacement with the St. Jude Toronto stentless porcine valve[J]. Ann Thorac Surg, 2004,78(6):2076-2083.
doi: 10.1016/j.athoracsur.2004.05.061 |
| [29] |
Flameng W, Meuris B, Herijgers P, et al. Prosjournal-patient mismatch is not clinically relevant in aortic valve replacement using the Carpentier-Edwards Perimount valve[J]. Ann Thorac Surg, 2006,82(2):530-536.
doi: 10.1016/j.athoracsur.2006.03.089 pmid: 16863756 |
| [30] |
Forcillo J, Pellerin M, Perrault LP, et al. Carpentier-Edwards pericardial valve in the aortic position: 25-years experience[J]. Ann Thorac Surg, 2013,96(2):486-493.
doi: 10.1016/j.athoracsur.2013.03.032 |
| [31] |
Gansera B, Hapfelmeier A, Brandl K, et al. The Mosaic bioprosjournal in the aortic position: 17 years’ results[J]. Thorac Cardiov Surg, 2014,62(1):26-34.
doi: 10.1055/s-0033-1345724 |
| [32] |
Gao G, Wu Y, Grunkemeier GL, et al. Durability of pericardial versus porcine aortic valves[J]. J Am Coll Cardiol, 2004,44(2):384-388.
doi: 10.1016/j.jacc.2004.01.053 pmid: 15261935 |
| [33] |
Glaser N, Franco-Cereceda A, Sartipy U. Late survival after aortic valve replacement with the perimount versus the Mosaic bioprosjournal[J]. Ann Thorac Surg, 2014,97(4):1314-1320.
doi: 10.1016/j.athoracsur.2013.10.078 |
| [34] |
Grunkemeier GL, Furnary AP, Wu Y, et al. Durability of pericardial versus porcine bioprosthetic heart valves[J]. J Thorac Cardiov Sur, 2012,144(6):1381-1386.
doi: 10.1016/j.jtcvs.2012.08.060 |
| [35] |
Guenzinger R, Fiegl K, Wottke M, et al. Twenty-seven-year experience with the St. Jude medical biocor bioprosjournal in the aortic position[J]. Ann Thorac Surg, 2015,100(6):2220-2226.
doi: 10.1016/j.athoracsur.2015.06.027 pmid: 26421496 |
| [36] |
Jamieson WR, Lemieux MD, Sullivan JA, et al. Medtronic intact porcine bioprosjournal experience to twelve years[J]. Ann Thorac Surg, 2001,71(5 Suppl):S278-281.
doi: 10.1016/s0003-4975(01)02548-6 pmid: 11388204 |
| [37] |
Jamieson WRE, Burr LH, Miyagishima RT, et al. Carpentier-Edwards supra-annular aortic porcine bioprosjournal: Clinical perfor-mance over 20 years[J]. J Thorac Cardiov Sur, 2005,130(4):994-1000.
doi: 10.1016/j.jtcvs.2005.03.040 |
| [38] |
Jamieson WRE, Koerfer R, Yankah CA, et al. Mitroflow aortic pericardial bioprosjournal—clinical performance[J]. Eur J Cardiothorac Surg, 2009,36(5):818-824.
doi: 10.1016/j.ejcts.2009.05.020 pmid: 19700338 |
| [39] |
Johnston DR, Soltesz EG, Vakil N, et al. Long-term durability of bioprosthetic aortic valves: implications from 12569 implants[J]. Ann Thorac Surg, 2015,99(4):1239-1247.
doi: 10.1016/j.athoracsur.2014.10.070 pmid: 25662439 |
| [40] | Kurlansky PA, Williams DB, Traad EA, et al. Surgical management of aortic valve disease in elderly patients with and without coronary artery disease: influence on quality of life[J]. J Cardiovasc Surg, 2007,48(2):215-226. |
| [41] |
Lootens L, Verbeke J, Martens T, et al. Ten-year results of aortic valve replacement with first-generation Mitroflow bioprosjournal: Is early degeneration a structural or a technical issue?[J]. Eur J Cardiothorac Surg, 2017,52(2):272-278.
doi: 10.1093/ejcts/ezx117 pmid: 28430883 |
| [42] |
Luciani GB, Santini F, Auriemma S, et al. Long-term results after aortic valve replacement with the Biocor PSB stentless xenograft in the elderly[J]. Ann Thorac Surg, 2001,71(5 Suppl):S306-310.
doi: 10.1016/s0003-4975(01)02525-5 pmid: 11388211 |
| [43] |
McClure RS, McGurk S, Cevasco M, et al. Late outcomes comparison of nonelderly patients with stented bioprosthetic and mechanical valves in the aortic position: a propensity-matched analysis[J]. J Thorac Cardiov Sur, 2014,148(5):1931-1939.
doi: 10.1016/j.jtcvs.2013.12.042 |
| [44] |
Minakata K, Tanaka S, Okawa Y, et al. Long-term outcome of the carpentier-edwards pericardial valve in the aortic position in Japanese patients[J]. Circ J, 2014,78(4):882-889.
doi: 10.1253/circj.CJ-13-1068 |
| [45] |
Minami K, Zittermann A, Schulte-Eistrup S, et al. Mitroflow synergy prostheses for aortic valve replacement: 19 years expe-rience with 1,516 patients[J]. Ann Thorac Surg, 2005,80(5):1699-1705.
doi: 10.1016/j.athoracsur.2005.04.053 pmid: 16242441 |
| [46] |
Mohammadi S, Kalavrouziotis D, Voisine P, et al. Bioprosthetic valve durability after stentless aortic valve replacement: the effect of implantation technique[J]. Ann Thorac Surg, 2014,97(6):2011-2018.
doi: 10.1016/j.athoracsur.2014.02.040 |
| [47] |
Mykén PSU, Bech-Hansen O. A 20-year experience of 1712 patients with the Biocor porcine bioprosjournal[J]. J Thorac Cardiov Sur, 2009,137(1):76-81.
doi: 10.1016/j.jtcvs.2008.05.068 |
| [48] |
Nishida T, Sonoda H, Oishi Y, et al. Long-term results of aortic valve replacement with mechanical prosjournal or carpentier-edwards perimount bioprosjournal in Japanese patients according to age[J]. Circ J, 2014,78(11):2688-2695.
doi: 10.1253/circj.CJ-14-0466 |
| [49] |
Pavoni D, Badano LP, Ius F, et al. Limited long-term durability of the Cryolife O’Brien stentless porcine xenograft valve[J]. Circulation, 2007,116(11 Suppl):I307-313.
doi: 10.1161/CIRCULATIONAHA.107.688564 pmid: 17846322 |
| [50] |
Kappetein AP, Puvimanasinghe JPA, Takkenberg JJM, et al. Predicted patient outcome after aortic valve replacement with Medtro-nic Stentless Freestyle bioprostheses[J]. J Heart Valve Dis, 2007,16(4):423-429.
pmid: 17702369 |
| [51] |
Repossini A, Fischlein T, Santarpino G, et al. Pericardial stentless valve for aortic valve replacement: long-term results[J]. Ann Thorac Surg, 2016,102(6):1956-1965.
doi: 10.1016/j.athoracsur.2016.05.080 pmid: 27544291 |
| [52] | Riess FC, Fradet G, Lavoie A, et al. Long-term outcomes of the Mosaic bioprosjournal[J]. Ann Thorac Surg, 2018,105(3):7763-7769. |
| [53] | Rizzoli G, Mirone S, Ius P, et al. Fifteen-year results with the Hancock Ⅱ valve: A multicenter experience [J]. J Thorac Car-diov Sur, 2006, 132(3): 602-609.e4. |
| [54] |
Ruggieri VG, Flecher E, Anselmi A, et al. Long-term results of the carpentier-edwards supraannular aortic valve prosjournal[J]. Ann Thorac Surg, 2012,94(4):1191-1197.
doi: 10.1016/j.athoracsur.2012.05.003 |
| [55] |
Sjögren J, Gudbjartsson T, Thulin LI. Long-term outcome of the mitroflow pericardial bioprosjournal in the elderly after aortic valve replacement[J]. J Heart Valve Dis, 2006,15(2):197-202.
pmid: 16607900 |
| [56] | Sponga S, Barbera MD, Pavoni D, et al. Ten-year results of the Freedom Solo stentless heart valve: excellent haemodynamics but progressive valve dysfunction in the long term[J]. Interact Cardiov Th, 2017,24(5):663-669. |
| [57] |
Stanger O, Bleuel I, Gisler F, et al. The Freedom Solo pericardial stentless valve: Single-center experience, outcomes, and long-term durability[J]. J Thorac Cardiov Sur, 2015,150(1):70-77.
doi: 10.1016/j.jtcvs.2015.01.060 |
| [58] | Wang Y, Chen S, Shi J, et al. Mid- to long-term outcome comparison of the Medtronic Hancock Ⅱ and bi-leaflet mechanical aortic valve replacement in patients younger than 60 years of age: a propensity-matched analysis[J]. Interact Cardiov Th, 2016,22(3):280-286. |
| [59] |
Webb J, Parkin D, Tøndel K, et al. A comparison of early redo surgery rates in Mosaic porcine and Perimount bovine pericardial valves[J]. Eur J Cardiothorac Surg, 2018,54(4):724-728.
doi: 10.1093/ejcts/ezy113 pmid: 29579171 |
| [60] |
Wilbring M, Alexiou K, Schumann E, et al. Isolated aortic valve replacement in patients with small aortic annulus-a high-risk group on long-term follow-up[J]. Thorac Cardiov Surg, 2013,61(5):379-385.
doi: 10.1055/s-00000085 |
| [61] |
Zibdeh O, Bugg I, Patel S, et al. Randomized trial of the Carpentier-Edwards supra-annular prosjournal versus the Medtronic Mosaic aortic prosjournal: 10-year results[J]. Eur J Cardiothorac Surg, 2018,54(2):281-287.
doi: 10.1093/ejcts/ezx512 pmid: 29401266 |
| [62] |
Head SJ, Celik M, Kappetein AP. Mechanical versus bioprosthe-tic aortic valve replacement[J]. Eur Heart J, 2017,38(28):2183-2191.
doi: 10.1093/eurheartj/ehx141 pmid: 28444168 |
| [63] |
Rodriguez-Gabella T, Voisine P, Puri R, et al. Aortic bioprosthetic valve durability: incidence, mechanisms, predictors, and management of surgical and transcatheter valve degeneration[J]. J Am Coll Cardiol, 2017,70(8):1013-1028.
doi: 10.1016/j.jacc.2017.07.715 pmid: 28818190 |
| [64] |
Schoen FJ, Levy RJ. Calcification of tissue heart valve substitutes: progress toward understanding and prevention[J]. Ann Thorac Surg, 2005,79(3):1072-1080.
doi: 10.1016/j.athoracsur.2004.06.033 pmid: 15734452 |
| [65] |
Siddiqui RF, Abraham JR, Butany J. Bioprosthetic heart valves: modes of failure[J]. Histopathology, 2009,55(2):135-144.
doi: 10.1111/j.1365-2559.2008.03190.x pmid: 19694820 |
| [66] |
Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease[J]. Eur Heart J, 2017,38(36):2739-2791.
doi: 10.1093/eurheartj/ehx391 pmid: 28886619 |
| [67] |
Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines[J]. J Am Coll Cardiol, 2017,70(2):252-289.
doi: 10.1016/j.jacc.2017.03.011 pmid: 28315732 |
| [68] |
Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients[J]. N Engl J Med, 2016,374(17):1609-1620.
doi: 10.1056/NEJMoa1514616 pmid: 27040324 |
| [69] |
Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial[J]. Lancet, 2015,385(9986):2477-2484.
doi: 10.1016/S0140-6736(15)60308-7 pmid: 25788234 |
| [70] |
Reardon MJ, van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients[J]. N Engl J Med, 2017,376(14):1321-1331.
pmid: 28304219 |
| [71] |
Karangelis D, Mazine A, Roubelakis A, et al. What is the role of sutureless aortic valves in today’s armamentarium?[J]. Expert Rev Cardiovasc Ther, 2017,15(2):83-91.
doi: 10.1080/14779072.2017.1273108 pmid: 27977305 |
| [1] | Ya-nan ZHAO,Hui-yun FAN,Xiang-yu WANG,Ya-nan LUO,Rong ZHANG,Xiao-ying ZHENG. Early death and causes of death of patients with autism spectrum disorders: A systematic review [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 375-383. |
| [2] | YUAN Chang-wei,WANG Ying-jin,ZHANG Shu-jie,SHEN Sheng-li,DUAN Hong-zhou. Clinical outcomes following microsurgery and endovascular embolization in the management of spinal dural arteriovenous fistula: A meta-analysis study [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 304-314. |
| [3] | FENG Jing-nan,GAO Le,SUN Yi-xin,YANG Ji-chun,DENG Si-wei,SUN Feng,ZHAN Si-yan. Accuracy of Xpert®MTB/RIF for the detection of tuberculosis and rifampicin-resistance tuberculosis in China: A systematic review and meta-analysis [J]. Journal of Peking University (Health Sciences), 2021, 53(2): 320-326. |
| [4] | Le GAO,Shu-qing YU,Ji-chun YANG,Jun-ling MA,Si-yan ZHAN,Feng SUN. Quality assessment of global guidelines on colorectal cancer screening [J]. Journal of Peking University(Health Sciences), 2019, 51(3): 548-555. |
| [5] | Yi-ran ZHANG,Feng RAO,Wei PI,Pei-xun ZHANG,Bao-guo JIANG. Proximal femoral nails antirotation and dynamic hip screws for fixation of unstable intertrochanteric fractures of femur: A meta-analysis [J]. Journal of Peking University(Health Sciences), 2019, 51(3): 493-500. |
| [6] | Shuang LIU,Yu-long GUO,Jing-yi YANG,Wei WANG,Jian XU. Efficacy of mesenchymal stem cells on systemic lupus erythematosus:a meta-analysis [J]. Journal of Peking University(Health Sciences), 2018, 50(6): 1014-1021. |
| [7] | SHI Hui-feng, ZHANG Jing-xu, ZHANG Rong, WANG Xiao-li. Prevalence of autism spectrum disorders in children aged 0-6 years in China: a meta-analysis [J]. Journal of Peking University(Health Sciences), 2017, 49(5): 798-806. |
| [8] | MA Yan-yan, ZHANG Jing-jing, GAO Xue-mei. Treatment outcome evaluation of different mandibular advancements using oral appliance to treat obstructive sleep apnea and hyponea syndrome: a systematic review [J]. Journal of Peking University(Health Sciences), 2017, 49(4): 691-699. |
| [9] | LI Zhi-xia, WU Shan-shan, YANG Zhi-rong, ZHAN Si-yan, SUN Feng. Impact of glucagon-like peptide-1 receptor agonists on nasopharyngitis and upper respiratory tract infection among patients with type 2 diabetes: a network meta-analysis [J]. Journal of Peking University(Health Sciences), 2016, 48(3): 454-459. |
| [10] | HUANG Yuan-Sheng, YANG Zhi-Rong, ZHAN Si-Yan. Comparison of simple pooling and bivariate model used in meta-analyses of diagnostic test accuracy published in Chinese journals [J]. Journal of Peking University(Health Sciences), 2015, 47(3): 483-488. |
| [11] | LIU Da-Jin, FENG Meng-Xian, LIU Min. Primary drug resistance of human immunodeficiency virus (HIV)among the treatment-naive individuals with HIV in China: a meta-analysis [J]. Journal of Peking University(Health Sciences), 2015, 47(3): 474-482. |
| [12] | WU Shan-Shan, ZHANG Yue-Lun, WANG Wei-Wei, CHEN Ru, SUN Feng, ZHAN Si-Yan- . Liver injury associated with treatment of multidrug-resistant tuberculosis: a syste-matic review and metaanalysis [J]. Journal of Peking University(Health Sciences), 2014, 46(3): 417-423. |
|
||