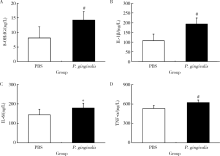
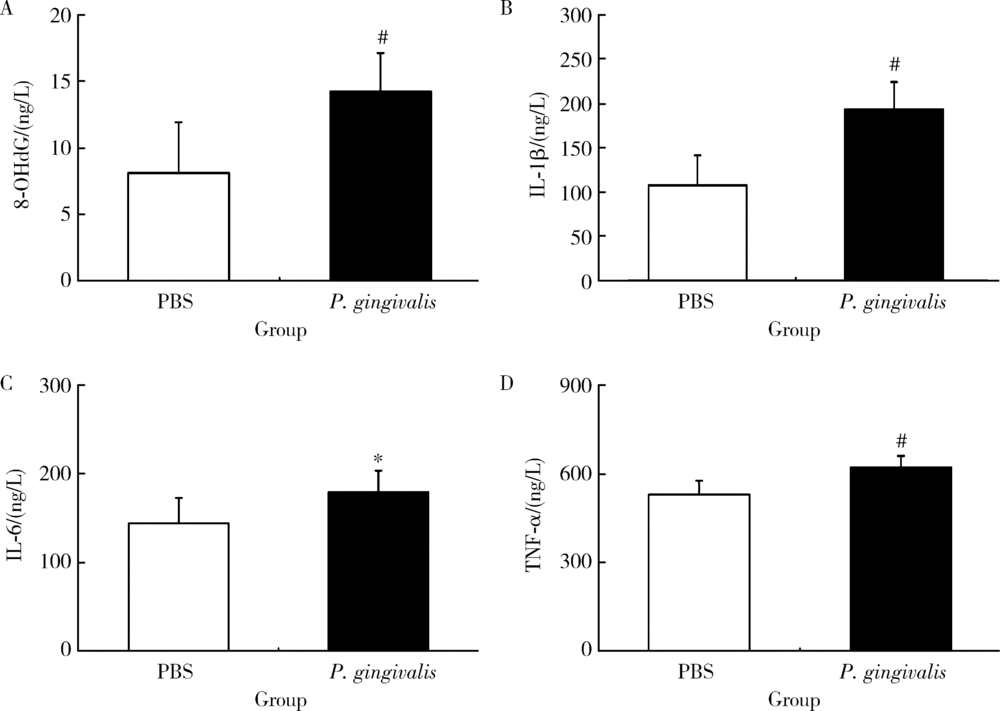
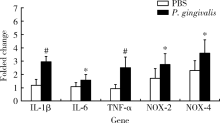
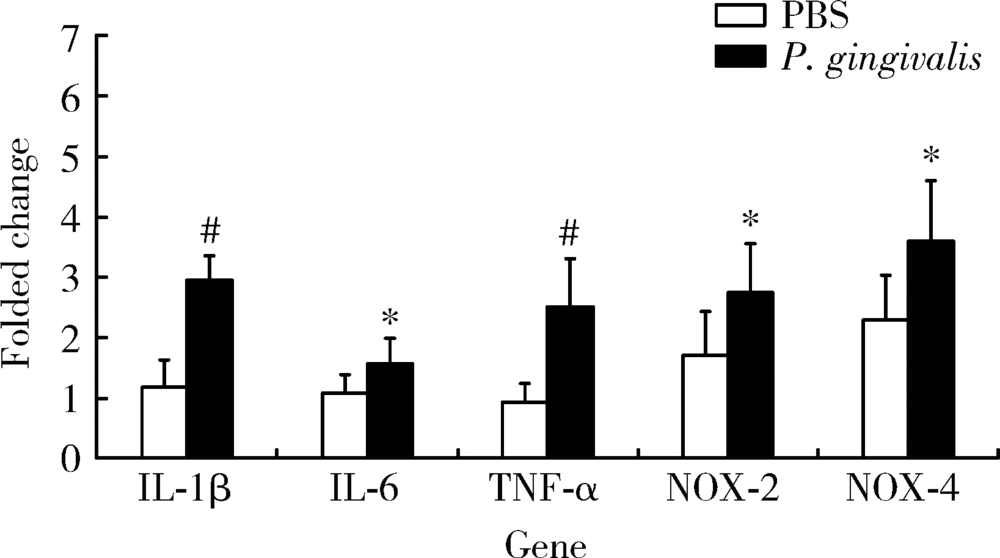
Journal of Peking University (Health Sciences) ›› 2020, Vol. 52 ›› Issue (4): 743-749. doi: 10.19723/j.issn.1671-167X.2020.04.028
Previous Articles Next Articles
Effect of Porphyromonas gingivalis infection on atherosclerosis in apolipoprotein-E knockout mice
Yan XUAN1,Yu CAI2,Xiao-xuan WANG2,Qiao SHI2,Li-xin QIU1,△( ),Qing-xian LUAN2,△(
),Qing-xian LUAN2,△( )
)
- 1. Fourth Clinical Division, Peking University School and Hospital of Stomatology, Beijing 100081, China
2. Department of Periodontology, Peking University School and Hospital of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
CLC Number:
- R781.42
| [1] | Cutler CW, Kalmar JR, Genco CA. Pathogenic strategies of the oral anaerobe, Porphyromonas gingivalis[J]. Trends Microbiol, 1995,3(2):45-51. |
| [2] | Holt SC, Kesavalu L, Walker S, et al. Virulence factors of Porphyromonas gingivalis[J]. Periodontol 2000,1999(20):168-238. |
| [3] | DeStefano F, Anda RF, Kahn HS, et al. Dental disease and risk of coronary heart disease and mortality[J]. BMJ, 1993,306(6879):688-691. |
| [4] | Southerland JH, Taylor GW, Moss K, et al. Commonality in chronic inflammatory diseases: Periodontitis, diabetes, and coronary artery disease[J]. Periodontol 2000, 2006,40(1):130-143. |
| [5] |
Dogan B, Buduneli E, Emingil G, et al. Characteristics of periodontal microflora in acute myocardial infarction[J]. J Periodontol, 2005,76(5):740-748.
pmid: 15898935 |
| [6] |
Jain A, Batista EL Jr, Serhan C, et al. Role for periodontitis in the progression of lipid deposition in an animal model[J]. Infect Immun, 2003,71(10):6012-6018.
doi: 10.1128/iai.71.10.6012-6018.2003 pmid: 14500522 |
| [7] | Ishihara K, Nabuchi A, Ito R, et al. Correlation between detection rates of periodontopathic bacterial DNA in carotid coronary stenotic artery plaque and in dental plaque samples[J]. J Clin Microbiol, 2004,42(3):1313-1315. |
| [8] | Nakano K, Inaba H, Nomura R, et al. Distribution of Porphyromonas gingivalis fimA genotypes in cardiovascular specimens from Japanese patients[J]. Oral Microbiol Immunol, 2008,23(2):170-172. |
| [9] | Kozarov EV, Dorn BR, Shelburne CE, et al. Human atherosclerotic plaque contains viable invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis[J]. Arterioscler Thromb Vasc Biol, 2005,25(3):e17-e18. |
| [10] |
Fukasawa A, Kurita-Ochiai T, Hashizume T, et al. Porphyromonas gingivalis accelerates atherosclerosis in C57BL/6 mice fed a high-fat diet[J]. Immunopharmacol Immunotoxicol, 2012,34(3):470-476.
doi: 10.3109/08923973.2011.627866 pmid: 22047042 |
| [11] |
Yu H, Qi LT, Liu LS, et al. Association of carotid intima-media thickness and atherosclerotic plaque with periodontal status[J]. J Dent Res, 2014,93(8):744-751.
doi: 10.1177/0022034514538973 pmid: 24935064 |
| [12] | Lin G, Chen S, Lei L, et al. Effects of intravenous injection of Porphyromonas gingivalis on rabbit inflammatory immune response and atherosclerosis [J/OL]. Mediators Inflamm, 2015: 364391. doi: 10.1155/2015/364391. |
| [13] |
Paigen B, Morrow A, Holmes PA, et al. Quantitative assessment of atherosclerotic lesions in mice[J]. Atherosclerosis, 1987,68(3):231-240.
pmid: 3426656 |
| [14] | Bélanger M, Rodrigues PH, Dunn WA, et al. Autophagy: A highway for Porphyromonas gingivalis in endothelial cells[J]. Autophagy, 2006,2(3):165-170. |
| [15] | Iwai T. Periodontal bacteremia and various vascular diseases[J]. J Periodontal Res, 2009,44(6):689-694. |
| [16] |
Campbell LA, Rosenfeld ME. Infection and atherosclerosis deve-lopment[J]. Arch Med Res, 2015,46(5):339-350.
doi: 10.1016/j.arcmed.2015.05.006 pmid: 26004263 |
| [17] |
Mattila KJ, Nieminen MS, Valtonen VV, et al. Association between dental health and acute myocardial infarction[J]. BMJ, 1989,298(6676):779-781.
doi: 10.1136/bmj.298.6676.779 pmid: 2496855 |
| [18] |
Chiu B. Multiple infections in carotid atherosclerotic plaques[J]. Am Heart J, 1999,138(5 Pt 2):S534-S536.
pmid: 10539867 |
| [19] | Haraszthy VI, Zambon JJ, Trevisan M, et al. Identification of periodontal pathogens in atheromatous plaques[J]. J Periodontol, 2000,71(10):1554-1560. |
| [20] |
Nakano K, Miyamoto E, Tamura K, et al. Distribution of 10 periodontal bacterial species in children and adolescents over a 7-year period[J]. Oral Dis, 2008,14(7):658-664.
doi: 10.1111/j.1601-0825.2008.01452.x pmid: 18565147 |
| [21] |
Wada K, Kamisaki Y. Roles of oral bacteria in cardiovascular diseases. From molecular mechanisms to clinical cases: Involvement of Porphyromonas gingivalis in the development of human aortic aneurysm[J]. J Pharmacol Sci, 2010,113(2):115-119.
doi: 10.1254/jphs.09r22fm pmid: 20501967 |
| [22] | Miyakawa H, Honma K, Qi M, et al. Interaction of Porphyromonas gingivalis with low-density lipoproteins: implications for a role for periodontitis in atherosclerosis[J]. J Periodontal Res, 2004,39(1):1-9. |
| [23] |
Li XY, Wang C, Xiang XR, et al. Porphyromonas gingivalis lipopolysaccharide increases lipid accumulation by affecting CD36 and ATP-binding cassette transporter A1 in macrophages[J]. Oncol Rep, 2013,30(3):1329-1336.
pmid: 23835648 |
| [24] |
Qi M, Miyakawa H, Kuramitsu HK. Porphyromonas gingivalis induces murine macrophage foam cell formation[J]. Microb Pathog, 2003,35(6):259-267.
pmid: 14580389 |
| [25] | Ross R. Atherosclerosis is an inflammatory disease[J]. Am Heart J, 1999,138(5 Pt 2):S419-S420. |
| [26] |
Hansson GK. Inflammation and immune response in atherosclerosis[J]. Curr Atheroscler Rep, 1999,1(2):150-155.
pmid: 11122704 |
| [27] |
Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis[J]. Nature, 2011,473(7347):317-325.
pmid: 21593864 |
| [28] |
Ramji DP, Davies TS. Cytokines in atherosclerosis: Key players in all stages of disease and promising therapeutic targets[J]. Cytokine Growth Factor Rev, 2015,26(6):673-685.
doi: 10.1016/j.cytogfr.2015.04.003 pmid: 26005197 |
| [29] |
Rosenson RS, Elliott M, Stasiv Y, et al. Randomized trial of an inhibitor of secretory phospholipase A2 on atherogenic lipoprotein subclasses in statin-treated patients with coronary heart disease[J]. Eur Heart J, 2011,32(8):999-1005.
doi: 10.1093/eurheartj/ehq374 pmid: 21081550 |
| [30] |
Hayashi C, Papadopoulos G, Gudino CV, et al. Protective role for TLR4 signaling in atherosclerosis progression as revealed by infection with a common oral pathogen[J]. J Immunol, 2012,189(7):3681-3688.
doi: 10.4049/jimmunol.1201541 pmid: 22956579 |
| [31] | Gibson FC 3rd, Ukai T, Genco CA. Engagement of specific innate immune signaling pathways during Porphyromonas gingivalis induced chronic inflammation and atherosclerosis[J]. Front Biosci, 2008(13):2041-2059. |
| [32] |
Pan S, Lei L, Chen S, et al. Rosiglitazone impedes Porphyromonas gingivalis-accelerated atherosclerosis by downregulating the TLR/NF-κB signaling pathway in atherosclerotic mice[J]. Int Immunopharmacol, 2014,23(2):701-708.
doi: 10.1016/j.intimp.2014.10.026 pmid: 25445963 |
| [33] |
Huang CY, Shih CM, Tsao NW, et al. The GroEL protein of Porphyromonas gingivalis regulates atherogenic phenomena in endothelial cells mediated by up-regulating toll-like receptor 4 expression[J]. Am J Transl Res, 2016,8(2):384-404.
pmid: 27158334 |
| [34] |
Khlgatian M, Nassar H, Chou HH, et al. Fimbria-dependent activation of cell adhesion molecule expression in Porphyromonas gingivalis-infected endothelial cells[J]. Infect Immun, 2002,70(1):257-267.
doi: 10.1128/iai.70.1.257-267.2002 pmid: 11748191 |
| [35] |
Gitlin JM, Loftin CD. Cyclooxygenase-2 inhibition increases lipopolysaccharide-induced atherosclerosis in mice[J]. Cardiovasc Res, 2009,81(2):400-407.
doi: 10.1093/cvr/cvn286 pmid: 18948273 |
| [36] |
Huang KT, Kuo L, Liao JC. Lipopolysaccharide activates endothelial nitric oxide synthase through protein tyrosine kinase[J]. Biochem Biophys Res Commun, 1998,245(1):33-37.
doi: 10.1006/bbrc.1998.8384 pmid: 9535778 |
| [37] |
Obermeier F, Gross V, Scholmerich J, et al. Interleukin-1 production by mouse macrophages is regulated in a feedback fashion by nitric oxide[J]. J Leukoc Biol, 1999,66(5):829-836.
pmid: 10577516 |
| [38] |
Sorescu D, Weiss D, Lassègue B. Superoxide production and expression of nox family proteins in human atherosclerosis[J]. Circulation, 2002,105(12):1429-1435.
doi: 10.1161/01.cir.0000012917.74432.66 pmid: 11914250 |
| [39] |
Szöcs K, Lassègue B, Sorescu D, et al. Upregulation of Nox-based NAD(P)H oxidases in restenosis after carotid injury[J]. Arterioscler Thromb Vasc Biol, 2002,22(1):21-27.
doi: 10.1161/hq0102.102189 pmid: 11788456 |
| [40] |
Anrather J, Racchumi G, Iadecola C. NF-kappaB regulates pha-gocytic NADPH oxidase by inducing the expression of gp91phox[J]. J Biol Chem, 2006,281(9):5657-5667.
doi: 10.1074/jbc.M506172200 pmid: 16407283 |
| [41] |
Morris KR, Lutz RD, Choi HS, et al. Role of the NF-kappaB signaling pathway and kappaB cis-regulatory elements on the IRF-1 and iNOS promoter regions in mycobacterial lipoarabinomannan induction of nitric oxide[J]. Infect Immun, 2003,71(3):1442-1452.
doi: 10.1128/iai.71.3.1442-1452.2003 pmid: 12595462 |
| [42] |
Deng WG, Zhu Y, Wu KK. Up-regulation of p300 binding and p50 acetylation in tumor necrosis factor-alpha-induced cyclooxygenase-2 promoter activation[J]. J Biol Chem, 2003,278(7):4770-4777.
doi: 10.1074/jbc.M209286200 pmid: 12471036 |
| [43] |
Brown K, Gerstberger S, Carlson L, et al. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation[J]. Science, 1995,267(5203):1485-1488.
doi: 10.1126/science.7878466 pmid: 7878466 |
| [44] |
Luoma JS, Stralin P, Marklund SL, et al. Expression of extracellular SOD and iNOS in macrophages and smooth muscle cells in human and rabbit atherosclerotic lesions: colocalization with epitopes characteristic of oxidized LDL and peroxynitrite-modified proteins[J]. Arterioscler Thromb Vasc Biol, 1998,18(2):157-167.
doi: 10.1161/01.atv.18.2.157 pmid: 9484979 |
| [45] |
Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-kappa B signaling[J]. Cell Res, 2011,21(1):103-115.
doi: 10.1038/cr.2010.178 pmid: 21187859 |
| [46] |
Stocker R, Keaney JF Jr. Role of oxidative modifications in atherosclerosis[J]. Physiol Rev, 2004,84(4):1381-1478.
doi: 10.1152/physrev.00047.2003 pmid: 15383655 |
| [1] | Jing HE,Zhongze FANG,Ying YANG,Jing LIU,Wenyao MA,Yong HUO,Wei GAO,Yangfeng WU,Gaoqiang XIE. Relationship between lipid metabolism molecules in plasma and carotid atheroscle-rotic plaques, traditional cardiovascular risk factors, and dietary factors [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 722-728. |
| [2] | Chang SHU,Ye HAN,Yuzhe SUN,Zaimu YANG,Jianxia HOU. Changes of parameters associated with anemia of inflammation in patients with stage Ⅲ periodontitis before and after periodontal initial therapy [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 45-50. |
| [3] | Chen-guang ZHANG,Xu-yan CHEN,Sheng WU,Li-li FENG,Yan WANG,Yu CHEN,Min DUAN,Ke WANG,Lin-lin SONG. Internal carotid artery pseudoaneurysm caused by parapharyngeal abscess: A case report [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1135-1138. |
| [4] | Xiu-rui LIANG,Xue-chun SHAN,Jing GUAN,Rui ZHANG,Jing YANG,Yi ZHANG,Jia-qi JIN,Yu-xin ZHANG,Fan XU,Ji-hua FU. Role of hyperglycemia-induced 5-hydroxytryptamine degradation of hepatic stellate cells in hepatic inflammation and fibrosis induced by type 2 diabetes mellitus [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1141-1150. |
| [5] | YUAN Lin-tian,MA Li-sha,LIU Run-yuan,QI wei,ZHANG Lu-dan,WANG Gui-yan,WANG Yu-guang. Computer simulation of molecular docking between methylene blue and some proteins of Porphyromonas gingivalis [J]. Journal of Peking University (Health Sciences), 2022, 54(1): 23-30. |
| [6] | BAI Feng,HE Yi-fan,NIU Ya-nan,YANG Ruo-juan,CAO Jing. Effects of ultrafine particulates on cardiac function in rat isolated heart [J]. Journal of Peking University (Health Sciences), 2021, 53(2): 240-245. |
| [7] | CHEN Huai-an,LIU Shuo,LI Xiu-jun,WANG Zhe,ZHANG Chao,LI Feng-qi,MIAO Wen-long. Clinical value of inflammatory biomarkers in predicting prognosis of patients with ureteral urothelial carcinoma [J]. Journal of Peking University (Health Sciences), 2021, 53(2): 302-307. |
| [8] | YANG Lin-cheng,ZHANG Rui-tao,GUO Li-jun,XIAO Han,ZU Ling-yun,ZHANG You-yi,CHENG Qin,ZHAO Zhi-ling,GE Qing-gang,GAO Wei. Hypoxia and inflammation are risk factors for acute myocardial injury in patients with coronavirus disease 2019 [J]. Journal of Peking University (Health Sciences), 2021, 53(1): 159-166. |
| [9] | Teng-fei LIU,Tao LIN,Li-hui REN,Guang-ping LI,Jian-jun PENG. Association between CMTM5 gene and coronary artery disease and the relative mechanism [J]. Journal of Peking University (Health Sciences), 2020, 52(6): 1082-1087. |
| [10] | Ping WANG,Jing SONG,Xiang-yu FANG,Xin LI,Xu LIU,Yuan JIA,Zhan-guo LI,Fan-lei HU. Role of erythroblast-like Ter cells in the pathogenesis of collagen-induced arthritis [J]. Journal of Peking University(Health Sciences), 2019, 51(3): 445-450. |
| [11] | Li-ping DUAN,Zhao-xia ZHENG,Yu-hui ZHANG,Jie DONG. Association of malnutrition-inflammation-cardiovascular disease with cognitive deterioration in peritoneal dialysis patients [J]. Journal of Peking University(Health Sciences), 2019, 51(3): 510-518. |
| [12] | Jing ZHANG,Su-fang LI,Hong CHEN,Jun-xian SONG. Role of miR-106b-5p in the regulation of gene profiles in endothelial cells [J]. Journal of Peking University(Health Sciences), 2019, 51(2): 221-227. |
| [13] | GAI Xiao-yan, CHANG Chun, WANG Juan, LIANG Ying, LI Mei-jiao, SUN Yong-chang,HE Bei, YAO Wan-zhen. Airway inflammation and small airway wall remodeling in neutrophilic asthma [J]. Journal of Peking University(Health Sciences), 2018, 50(4): 645-650. |
| [14] | WANG Hao, CHEN Liang, YE Xiao-yun. Triptolide induces oxidative stress and apoptosis and activates PIK3/Akt signaling pathway in TM4 sertoli cells [J]. Journal of Peking University(Health Sciences), 2018, 50(4): 607-612. |
| [15] | ZHANG Yi1, SONG Xiao-ming1, ZHAO Qian, WANG Tong, Li Li-juan, CHEN Jie, XU Hong-bing, LIU Bei-bei, SUN Xiao-yan, HE Bei, HUANG Wei. Effects of exposure to ambient particulate matter and polycyclic aromatic hydrocarbons on oxidative stress biomarkers in the patients with chronic obstructive pulmonary disease [J]. Journal of Peking University(Health Sciences), 2017, 49(3): 394-402. |
|
||