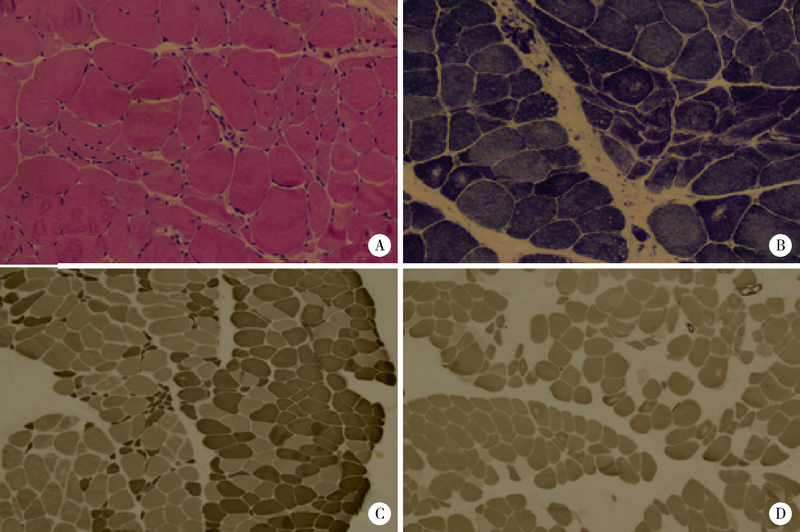
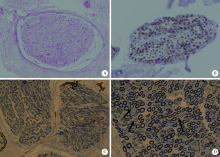
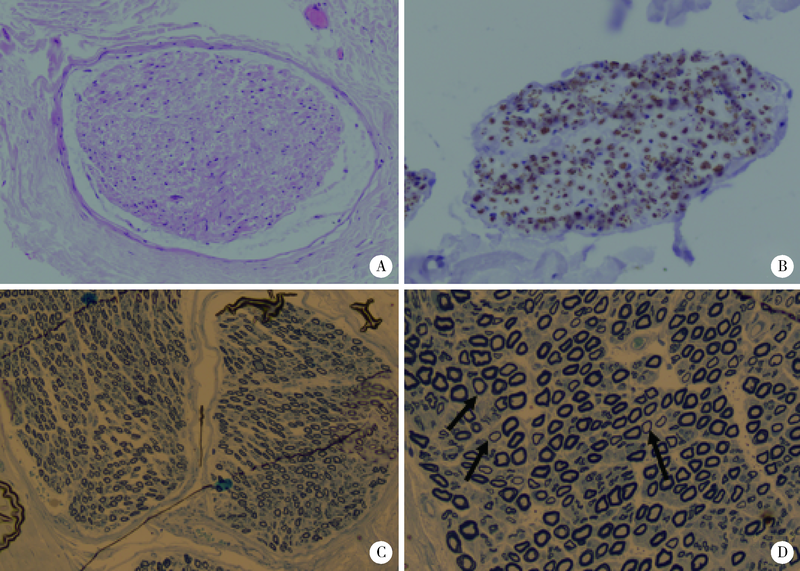
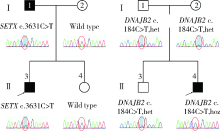
Journal of Peking University (Health Sciences) ›› 2021, Vol. 53 ›› Issue (5): 957-963. doi: 10.19723/j.issn.1671-167X.2021.05.025
Previous Articles Next Articles
Clinical, pathological and genetic characteristics of 8 patients with distal hereditary motor neuropathy
LIU Mei-ge1,FANG Pu2,WANG Yan1,CONG Lu1,FAN Yang-yi1,YUAN Yuan1,XU Yan1,ZHANG Jun1,HONG Dao-jun1,2,△( )
)
- 1. Department of Neurology, Peking University People’s Hospital, Beijing 100044, China
2. Department of Neurology, the First Affiliated Hospital of Nanchang University, Nanchang 330006, China
CLC Number:
- R596
| [1] |
Garg N, Park SB, Vucic S, et al. Differentiating lower motor neuron syndrome [J]. J Neurol Neurosurg Psychiatry, 2017, 88(6):474-483.
doi: 10.1136/jnnp-2016-313526 |
| [2] |
Frasquet M, Rojas-García R, Argente-Escrig H, et al. Distal hereditary motor neuropathies: mutation spectrum and genotype-phenotype correlation [J]. Eur J Neurol, 2021, 28(4):1334-1343.
doi: 10.1111/ene.14700 pmid: 33369814 |
| [3] |
Bansagi B, Griffin H, Whittaker RG, et al. Genetic heterogeneity of motor neuropathies [J]. Neurology, 2017, 88(13):1226-1234.
doi: 10.1212/WNL.0000000000003772 |
| [4] | Bacquet J, Stojkovic T, Boyer A, et al. Molecular diagnosis of inherited peripheral neuropathies by targeted next-generation sequencing: molecular spectrum delineation [J]. BMJ Open, 2018, 8(10):e21632. |
| [5] |
Echaniz-Laguna A, Geuens T, Petiot P, et al. Axonal neuropathies due to mutations in small heat shock proteins: clinical, genetic, and functional insights into novel mutations [J]. Hum Mutat, 2017, 38(5):556-568.
doi: 10.1002/humu.23189 pmid: 28144995 |
| [6] |
Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology [J]. Genet Med, 2015, 17(5):405-424.
doi: 10.1038/gim.2015.30 pmid: 25741868 |
| [7] |
Tanabe H, Higuchi Y, Yuan JH, et al. Clinical and genetic features of charcot-marie-tooth disease 2F and hereditary motor neuropathy 2B in Japan [J]. J Peripher Nerv Syst, 2018, 23(1):40-48.
doi: 10.1111/jns.2018.23.issue-1 |
| [8] |
Windpassinger C, Auer-Grumbach M, Irobi J, et al. Heterozygous missense mutations in BSCL2 are associated with distal hereditary motor neuropathy and Silver syndrome [J]. Nat Genet, 2004, 36(3):271-276.
doi: 10.1038/ng1313 |
| [9] |
Novarino G, Fenstermaker AG, Zaki MS, et al. Exome sequencing links corticospinal motor neuron disease to common neurodegenerative disorders [J]. Science, 2014, 343(6170):506-511.
doi: 10.1126/science.1247363 pmid: 24482476 |
| [10] | Xie Y, Lin Z, Pakhrin PS, et al. Genetic and clinical features in 24 Chinese distal hereditary motor neuropathy families [J/OL]. Front Neurol, 2020, 11:603003(2020-12-14)[2020-12-15]. https://pubmed-ncbi-nlm-nih-gov-443.webvpn.bjmu.edu.cn/33 3810781/ . |
| [11] | 张付峰, 卢晓琴, 严新翔, 等. 远端型遗传性运动神经病的临床特征分析 [J]. 第二军医大学学报, 2009, 30(1):57-60. |
| [12] |
De Jonghe P, Auer-Grumbach M, Irobi J, et al. Autosomal dominant juvenile amyotrophic lateral sclerosis and distal hereditary motor neuronopathy with pyramidal tract signs: synonyms for the same disorder [J]. Brain, 2002, 125(Pt 6):1320-1325.
pmid: 12023320 |
| [13] |
Motley WW, Griffin LB, Mademan I, et al. A novel AARS mutation in a family with dominant myeloneuropathy [J]. Neurology, 2015, 84(20):2040-2047.
doi: 10.1212/WNL.0000000000001583 |
| [14] |
Luigetti M, Fabrizi GM, Madia F, et al. Seipin S90L mutation in an Italian family with CMT2/dHMN and pyramidal signs [J]. Muscle Nerve, 2010, 42(3):448-451.
doi: 10.1002/mus.21734 pmid: 20806400 |
| [15] |
Beecroft SJ, McLean CA, Delatycki MB, et al. Expanding the phenotypic spectrum associated with mutations of DYNC1H1 [J]. Neuromuscul Disord, 2017, 27(7):607-615.
doi: 10.1016/j.nmd.2017.04.011 |
| [16] |
Dierick I, Baets J, Irobi J, et al. Relative contribution of mutations in genes for autosomal dominant distal hereditary motor neuropathies: a genotype-phenotype correlation study [J]. Brain, 2008, 131(Pt 5):1217-1227.
doi: 10.1093/brain/awn029 pmid: 18325928 |
| [17] |
Rossor AM, Evans MR, Reilly MM. A practical approach to the genetic neuropathies [J]. Pract Neurol, 2015, 15(3):187-198.
doi: 10.1136/practneurol-2015-001095 |
| [18] |
Liu X, Duan X, Zhang Y, Sun A, et al. Molecular analysis and clinical diversity of distal hereditary motor neuropathy [J]. Eur J Neurol, 2020, 27:1319-1326.
doi: 10.1111/ene.14260 pmid: 32298515 |
| [19] |
Beijer D, Baets J. The expanding genetic landscape of hereditary motor neuropathies [J]. Brain, 2020, 143(Pt 12):3540-3563.
doi: 10.1093/brain/awaa311 |
| [1] | Zhihui WU, Mingzhi HU, Qiaoying ZHAO, Fengfeng LV, Jingying ZHANG, Wei ZHANG, Yongfu WANG, Xiaolin SUN, Hui WANG. Immunomodulatory mechanism of umbilical cord mesenchymal stem cells modified by miR-125b-5p in systemic lupus erythematosus [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 860-867. |
| [2] | Jun WANG, Lan YAO, Ning ZHANG, Libin SUO, Hongpei LI, Yue WEI, Peng CHA, Zheng LIANG, Kunpeng LIU. Effects of unilateral thoracic paravertebal block on hemodynamic and the level of conscionsness during double lumen endotracheal intubation [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 890-895. |
| [3] | Dongwu LIU, Jie CHEN, Mingli GAO, Jing YU. Rheumatoid arthritis with Castleman-like histopathology in lymph nodes: A case report [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 928-931. |
| [4] | Shuhui YU,Jianing HAN,Lijun ZHONG,Congyu CHEN,Yunxiang XIAO,Yanbo HUANG,Yang YANG,Xinyan CHE. Predictive value of preoperative pelvic floor electrophysiological parameters on early urinary incontinence following radical prostatectomy [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 594-599. |
| [5] | Huangda GUO,Hexiang PENG,Siyue WANG,Tianjiao HOU,Yixin LI,Hanyu ZHANG,Mengying WANG,Yiqun WU,Xueying QIN,Xun TANG,Jing LI,Dafang CHEN,Yonghua HU,Tao WU. A ssociations of short-term ambient particulate matter exposure and MTNR1B gene with triglyceride-glucose index: A family-based study [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 375-383. |
| [6] | Han ZHAO,Yan WEI,Xuehui ZHANG,Xiaoping YANG,Qing CAI,Chengyun NING,Mingming XU,Wenwen LIU,Ying HUANG,Ying HE,Yaru GUO,Shengjie JIANG,Yunyang BAI,Yujia WU,Yusi GUO,Xiaona ZHENG,Wenjing LI,Xuliang DENG. Bionic design, preparation and clinical translation of oral hard tissue restorative materials [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 4-8. |
| [7] | Yuan PAN,Hang GU,Han XIAO,Lijun ZHAO,Yiman TANG,Wenshu GE. Ubiquitin-specific protease 42 regulates osteogenic differentiation of human adipose-derived stem cells [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 9-16. |
| [8] | Xiaoqiang LIU,Yin ZHOU. Risk factors of perioperative hypertension in dental implant surgeries with bone augmentation [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 93-98. |
| [9] | Deng-hui DUAN,Hom-Lay WANG,En-bo WANG. Role of collagen membrane in modified guided bone regeneration surgery using buccal punch flap approach: A retrospective and radiographical cohort study [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1097-1104. |
| [10] | Huan-rui LIU,Xiang PENG,Sen-lin LI,Xin GOU. Risk modeling based on HER-2 related genes for bladder cancer survival prognosis assessment [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 793-801. |
| [11] | Hui WEI, Ci-dan-yang-zong, Yi-xi-la-mu, Bai-ma-yang-jin. Risk factors associated with different types of Henoch-Schönlein purpura in Tibetan patients at high altitude [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 923-928. |
| [12] | Yin-ji JIN,Lin SUN,Jin-xia ZHAO,Xiang-yuan LIU. Significance of IgA isotype of anti-v-raf murine sarcoma viral oncogene homologue B1 antibody in rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 631-635. |
| [13] | Shang XIE,Zhi-gang CAI,Xiao-feng SHAN. Application value of whole exon sequencing and immune related indicators in the precision treatment of oral squamous cell carcinoma [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 697-701. |
| [14] | Yun-fei DAI,He LIU,Chu-fang PENG,Xi-jun JIANG. Retrospective evaluation of treatment outcomes in immature teeth treated with regenerative endodontic procedures with an over-36-month review [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 729-735. |
| [15] | Yuan-yuan XU,Zhi-lin SUN,Xiu-lian ZHANG,Zi-lian LIU,Wei LIU,Xin GUAN. Carbamazepine induced Stevens-Johnson syndrome in Han Chinese with positive HLA-A * 3101 gene: A case report [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 755-757. |
|
||