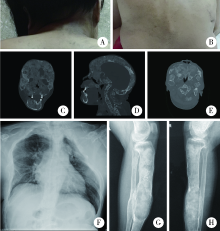
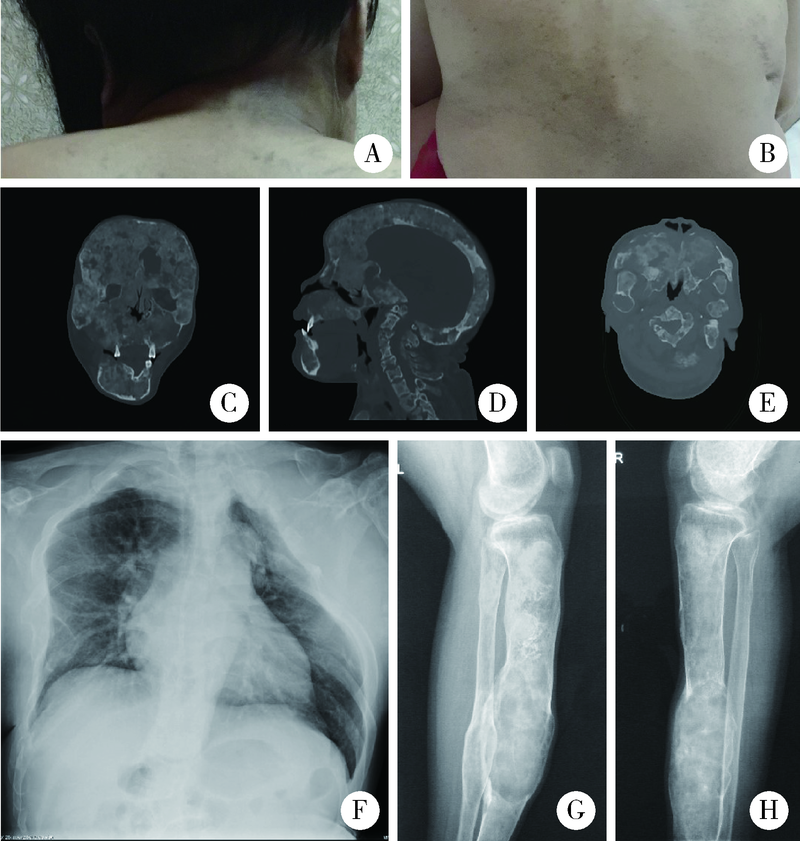
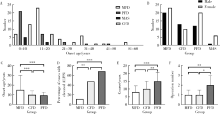
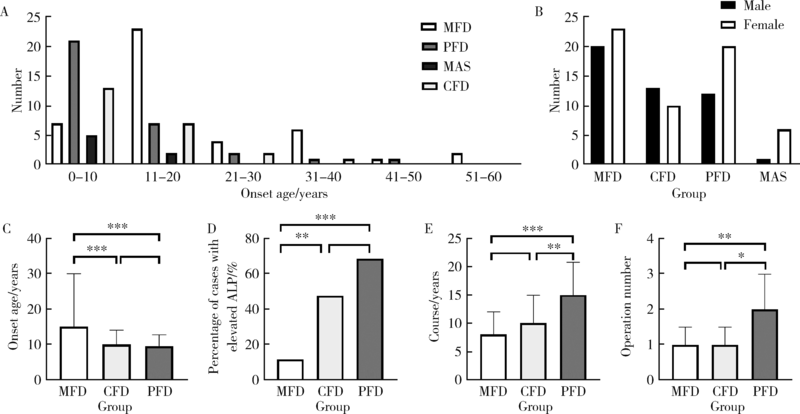
Journal of Peking University (Health Sciences) ›› 2022, Vol. 54 ›› Issue (1): 54-61. doi: 10.19723/j.issn.1671-167X.2022.01.009
Previous Articles Next Articles
Clinicopathological analysis of 105 patients with fibrous dysplasia of cranio-maxillofacial region
XUE Jiang1,ZHANG Jian-yun1,SHI Rui-rui2,XIE Xiao-yan3,BAI Jia-ying1,LI Tie-jun1,△( )
)
- 1. Department of Oral Pathology, Peking University School and Hospital of Stomatology, Beijing 100081, China
2. Central Laboratory, Peking University School and Hospital of Stomatology, Beijing 100081, China
3. Department of Oral and Maxillofacial Radiology, Peking University School and Hospital of Stomatology & National Center of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
CLC Number:
- R739.8
| [1] |
Dumitrescu CE, Collins MT. McCune-Albright syndrome[J]. Orphanet J Rare Dis, 2008, 3:12.
doi: 10.1186/1750-1172-3-12 pmid: 18489744 |
| [2] | 叶为民, 竺涵光, 郑家伟, 等. 46例颌面部骨纤维异常增殖症临床分析[J]. 中国口腔颌面外科杂志, 2008, 6(3):170-173. |
| [3] | El-Naggar AK, Chan JK, Grandis JR, et al. WHO classification of head and neck tumours [M]. Lyon: IARC Press, 2017: 253-254. |
| [4] | 张壁, 韩其滨, 赵吉宏, 等. 30例颌面部骨纤维异常增殖症诊治的临床分析[J]. 口腔医学研究, 2010, 26(5):713-715. |
| [5] |
Sweeney K, Kaban LB. Natural history and progression of craniofacial fibrous dysplasia: A retrospective evaluation of 114 patients from Massachusetts General Hospital[J]. J Oral Maxillofac Surg, 2020, 78(11):1966-1980.
doi: 10.1016/j.joms.2020.05.036 |
| [6] |
Cheng J, Wang Y, Yu H, et al. An epidemiological and clinical analysis of craniomaxillofacial fibrous dysplasia in a Chinese population[J]. Orphanet J Rare Dis, 2012, 7:80.
doi: 10.1186/1750-1172-7-80 pmid: 23074969 |
| [7] |
Javaid MK, Boyce A, Appelman-Dijkstra N, et al. Best practice management guidelines for fibrous dysplasia/McCune-Albright syndrome: A consensus statement from the FD/MAS international consortium[J]. Orphanet J Rare Dis, 2019, 14(1):139.
doi: 10.1186/s13023-019-1102-9 |
| [8] | World Health Organization. WHO classification of tumours of soft tissue and bone [M]. 5th ed. Lyon: IARC Press, 2020: 472-474. |
| [9] |
Ma J, Liang L, Gu B, et al. A retrospective study on craniofacial fibrous dysplasia: Preoperative serum alkaline phosphatase as a prognostic marker?[J]. J Craniomaxillofac Surg, 2013, 41(7):644-647.
doi: 10.1016/j.jcms.2012.12.007 |
| [10] | Chen YR, Wong FH, Hsueh C, et al. Computed tomography characteristics of non-syndromic craniofacial fibrous dysplasia[J]. Chang Gung Med J, 2002, 25(1):1-8. |
| [11] |
Burke AB, Collins MT, Boyce AM. Fibrous dysplasia of bone: Craniofacial and dental implications[J]. Oral Dis, 2017, 23(6):697-708.
doi: 10.1111/odi.12563 pmid: 27493082 |
| [12] |
Akintoye SO, Lee JS, Feimster T, et al. Dental characteristics of fibrous dysplasia and McCune-Albright syndrome[J]. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 2003, 96(3):275-282.
doi: 10.1016/S1079-2104(03)00225-7 |
| [13] |
Waldron CA. Fibro-osseous lesions of the jaws[J]. J Oral Maxillofac Surg, 1993, 51(8):828-835.
doi: 10.1016/S0278-2391(10)80097-7 |
| [14] |
Gupta D, Garg P, Mittal A. Computed tomography in craniofacial fibrous dysplasia: A case series with review of literature and classification update[J]. Open Dent J, 2017, 11:384-403.
doi: 10.2174/1874210601711010384 |
| [15] | 李铁军. 口腔组织学与病理学 [M]. 3版. 北京: 北京大学医学出版社, 2020: 360-361. |
| [16] |
Slootweg PJ. Maxillofacial fibro-osseous lesions: Classification and differential diagnosis[J]. Semin Diagn Pathol, 1996, 13(2):104-112.
pmid: 8734416 |
| [17] |
Riminucci M, Liu B, Corsi A, et al. The histopathology of fibrous dysplasia of bone in patients with activating mutations of the Gs alpha gene: Site-specific patterns and recurrent histological hallmarks[J]. J Pathol, 1999, 187(2):249-258.
pmid: 10365102 |
| [18] |
Sissons HA, Steiner GC, Dorfman HD. Calcified spherules in fibro-osseous lesions of bone[J]. Arch Pathol Lab Med, 1993, 117(3):284-290.
pmid: 8442673 |
| [19] | Dorfman HD. New knowledge of fibro-osseous lesions of bone[J]. Int J Surg Pathol, 2010, 18(3 Suppl):62S-65S. |
| [20] |
Sargolzaei S, Ghelejkhani A, Baghban AA. Diagnostic and bio-logical significance of immunohistochemical expression of osteopontin and Ki67 in fibro-osseous lesions of jaws[J]. J Islam Dent Assoc IRAN, 2017, 29(2):70-78.
doi: 10.30699/jidai.29.2.70 |
| [21] |
Shi RR, Li XF, Zhang R, et al. GNAS mutational analysis in differentiating fibrous dysplasia and ossifying fibroma of the jaw[J]. Mod Pathol, 2013, 26(8):1023-1031.
doi: 10.1038/modpathol.2013.31 |
| [22] |
Li Z, Raynald, Wang Z, et al. Malignant transformation of craniofacial fibrous dysplasia: A systematic review of overall survival[J]. Neurosurg Rev, 2020, 43(3):911-921.
doi: 10.1007/s10143-019-01089-1 |
| [1] | Dongwu LIU, Jie CHEN, Mingli GAO, Jing YU. Rheumatoid arthritis with Castleman-like histopathology in lymph nodes: A case report [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 928-931. |
| [2] | Yuxuan TIAN,Mingjian RUAN,Yi LIU,Derun LI,Jingyun WU,Qi SHEN,Yu FAN,Jie JIN. Predictive effect of the dual-parametric MRI modified maximum diameter of the lesions with PI-RADS 4 and 5 on the clinically significant prostate cancer [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 567-574. |
| [3] | Zhanhong LAI,Jiachen LI,Zelin YUN,Yonggang ZHANG,Hao ZHANG,Xiaoyan XING,Miao SHAO,Yuebo JIN,Naidi WANG,Yimin LI,Yuhui LI,Zhanguo LI. A unicenter real-world study of the correlation factors for complete clinical response in idiopathic inflammatory myopathies [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 284-292. |
| [4] | Xunmin XU,Xiao SHAO,Aiping JI. Analysis of death cases in the oral emergency department [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 185-189. |
| [5] | Lu FENG,Jia-yu ZHAI,Jin-xia ZHAO. Medical visit status and clinical features in patients with IgG4 related disease [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1028-1032. |
| [6] | Hui WEI, Ci-dan-yang-zong, Yi-xi-la-mu, Bai-ma-yang-jin. Risk factors associated with different types of Henoch-Schönlein purpura in Tibetan patients at high altitude [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 923-928. |
| [7] | Yun-fei SHI,Hao-jie WANG,Wei-ping LIU,Lan MI,Meng-ping LONG,Yan-fei LIU,Yu-mei LAI,Li-xin ZHOU,Xin-ting DIAO,Xiang-hong LI. Analysis of clinicopathological and molecular abnormalities of angioimmunoblastic T-cell lymphoma [J]. Journal of Peking University (Health Sciences), 2023, 55(3): 521-529. |
| [8] | Li-jia MA,Pan-pan HU,Xiao-guang LIU. Spinal metastases combined with leptomeningeal metastasis: A case report [J]. Journal of Peking University (Health Sciences), 2023, 55(3): 563-566. |
| [9] | Qi SHEN,Yi-xiao LIU,Qun HE. Mucinous tubular and spindle cell carcinoma of kidney: Clinicopathology and prognosis [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 276-282. |
| [10] | Wei-hua HOU,Shu-jie SONG,Zhong-yue SHI,Mu-lan JIN. Clinicopathological features of Helicobacter pylori-negative early gastric cancer [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 292-298. |
| [11] | Xue-mei HA,Yong-zheng YAO,Li-hua SUN,Chun-yang XIN,Yan XIONG. Solid placental transmogrification of the lung: A case report and literature review [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 357-361. |
| [12] | Bo-han NING,Qing-xia ZHANG,Hui YANG,Ying DONG. Endometrioid adenocarcinoma with proliferated stromal cells, hyalinization and cord-like formations: A case report [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 366-369. |
| [13] | Qian SU,Xin PENG,Chuan-xiang ZHOU,Guang-yan YU. Clinicopathological characteristics and prognosis of non-Hodgkin lymphoma in oral and maxillofacial regions: An analysis of 369 cases [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 13-21. |
| [14] | Wen-xin CAI,Shi-cheng LI,Yi-ming LIU,Ru-yu LIANG,Jing LI,Jian-ping GUO,Fan-lei HU,Xiao-lin SUN,Chun LI,Xu LIU,Hua YE,Li-zong DENG,Ru LI,Zhan-guo LI. A cross-sectional study on the clinical phenotypes of rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1068-1073. |
| [15] | Rui LIU,Jin-xia ZHAO,Liang YAN. Clinical characteristics of patients with rheumatoid arthritis complicated with venous thrombosis of lower extremities [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1079-1085. |
|
||