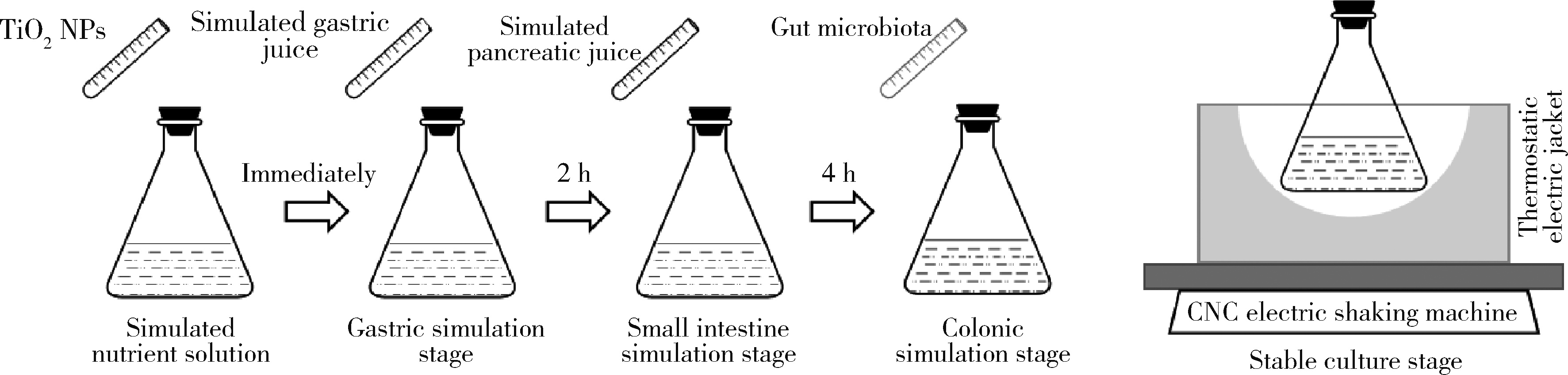
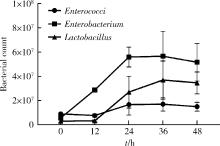
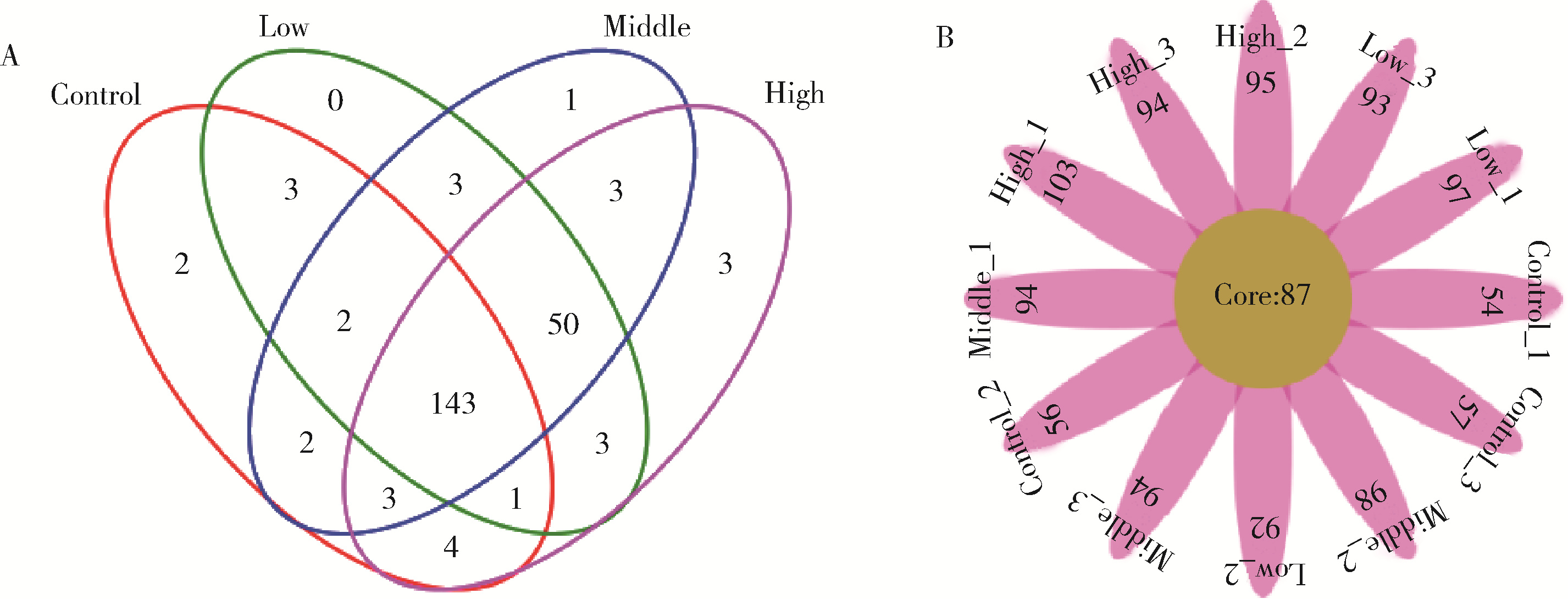
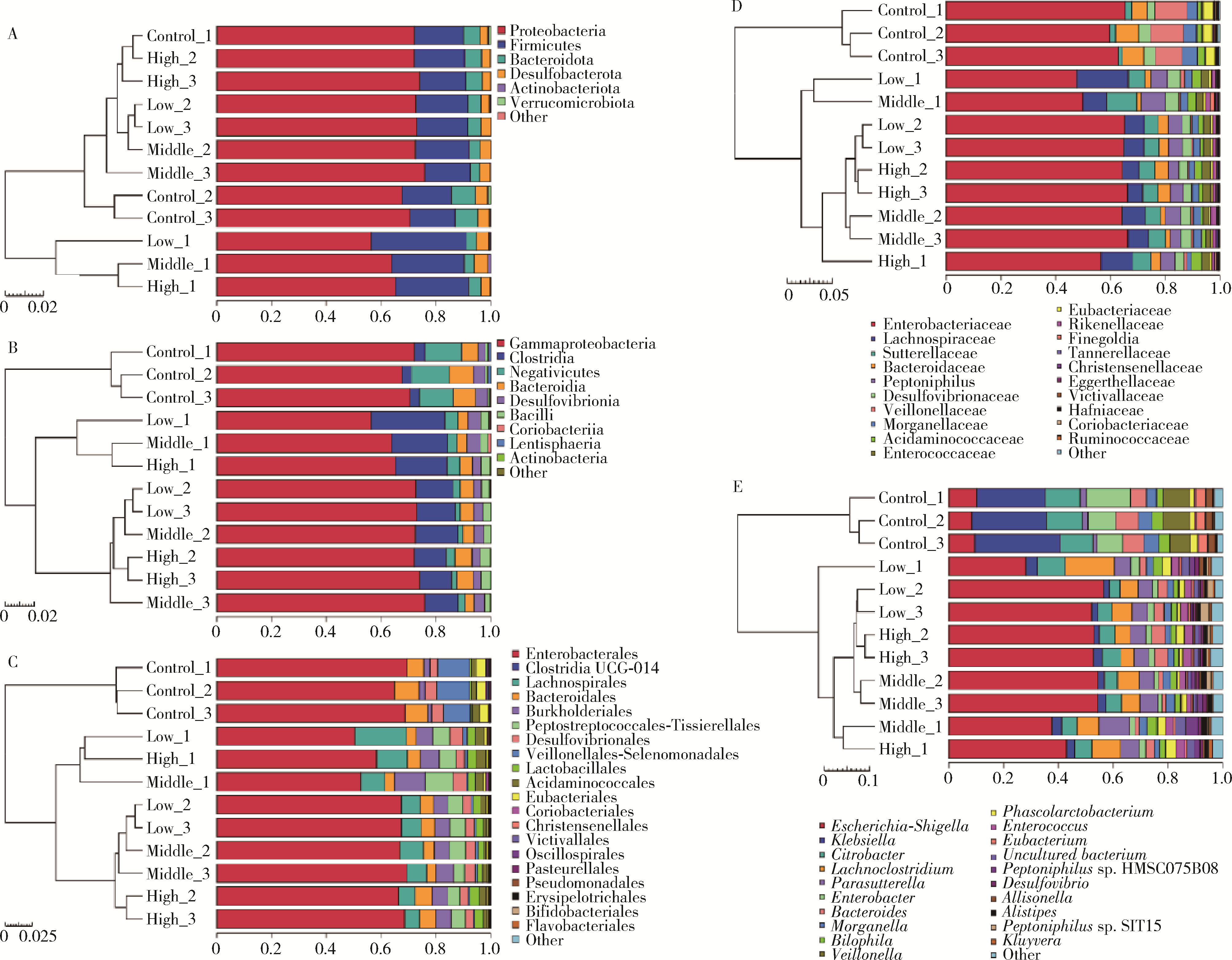
Journal of Peking University (Health Sciences) ›› 2022, Vol. 54 ›› Issue (3): 468-476. doi: 10.19723/j.issn.1671-167X.2022.03.011
Previous Articles Next Articles
Effects of nano titanium dioxide on gut microbiota based on human digestive tract microecology simulation system in vitro
Jia-he ZHANG,Jia-qi SHI,Zhang-jian CHEN,Guang JIA*( )
)
- Department of Occupational and Environmental Health Sciences, Peking University School of Public Health; Beijing Key Laboratory of Toxicological Research and Risk Assessment for Food Safety, Beijing 100191, China
CLC Number:
- R155.5
| 1 | Aguilar F, Crebelli R, Di Domenico A, et al. Re-evaluation of titanium dioxide (E 171) as a food additive[J/OL]. EFSA Journal, 2016, 14(9): 4545. https://doi.org/10.2903/j.efsa.2016.4545. |
| 2 |
Yang Y , Doudrick K , Bi X , et al. Characterization of food-grade titanium dioxide: The presence of nanosized particles[J]. Environ Sci Technol, 2014, 48 (11): 6391- 6400.
doi: 10.1021/es500436x |
| 3 |
Chen ZJ , Han S , Zhou SP , et al. Review of health safety aspects of titanium dioxide nanoparticles in food application[J]. Nanoimpact, 2020, 18, 100224.
doi: 10.1016/j.impact.2020.100224 |
| 4 |
Shakeel M , Jabeen F , Shabbir S , et al. Toxicity of nano-titanium dioxide (TiO2-NP) through various routes of exposure: A review[J]. Biol Trace Elem Res, 2016, 172 (1): 1- 36.
doi: 10.1007/s12011-015-0550-x |
| 5 | Alavi M , Karimi N . Characterization, antibacterial, total antioxidant, scavenging, reducing power and ion chelating activities of green synthesized silver, copper and titanium dioxide nanoparticles using Artemisia haussknechtii leaf extract[J]. Artif Cells Nanomed Biotechnol, 2018, 46 (8): 2066- 2081. |
| 6 |
Hajipour MJ , Fromm KM , Ashkarran AA , et al. Antibacterial properties of nanoparticles[J]. Trends Biotechnol, 2012, 30 (10): 499- 511.
doi: 10.1016/j.tibtech.2012.06.004 |
| 7 | Daou I , Moukrad N , Zegaoui O , et al. Antimicrobial activity of ZnO-TiO2 nanomaterials synthesized from three different precursors of ZnO: Influence of ZnO/TiO2 weight ratio[J]. Water Sci Technol, 2018, 77 (5/6): 1238- 1249. |
| 8 |
Chen L , Guo Y , Hu C , et al. Dysbiosis of gut microbiota by chronic coexposure to titanium dioxide nanoparticles and bisphenol A: Implications for host health in zebrafish[J]. Environ Pollut, 2018, 234, 307- 317.
doi: 10.1016/j.envpol.2017.11.074 |
| 9 |
Chen Z , Han S , Zhou D , et al. Effects of oral exposure to tita-nium dioxide nanoparticles on gut microbiota and gut-associated metabolism in vivo[J]. Nanoscale, 2019, 11 (46): 22398- 22412.
doi: 10.1039/C9NR07580A |
| 10 |
Chen Z , Zhou D , Han S , et al. Hepatotoxicity and the role of the gut-liver axis in rats after oral administration of titanium dioxide nanoparticles[J]. Part Fibre Toxicol, 2019, 16 (1): 48.
doi: 10.1186/s12989-019-0332-2 |
| 11 |
Mu W , Wang Y , Huang C , et al. Effect of long-term intake of dietary titanium dioxide nanoparticles on intestine inflammation in mice[J]. J Agric Food Chem, 2019, 67 (33): 9382- 9389.
doi: 10.1021/acs.jafc.9b02391 |
| 12 |
Li J , Yang S , Lei R , et al. Oral administration of rutile and anatase TiO2 nanoparticles shifts mouse gut microbiota structure[J]. Nanoscale, 2018, 10 (16): 7736- 7745.
doi: 10.1039/C8NR00386F |
| 13 | 刘倩, 姜建辉, 吴瑛. 纳米二氧化钛对果蝇肠道共生菌的影响[J]. 黑龙江农业科学, 2017, (9): 94- 97. |
| 14 |
Brodkorb A , Egger L , Alminger M , et al. INFOGEST static in vitro simulation of gastrointestinal food digestion[J]. Nat Protoc, 2019, 14 (4): 991- 1014.
doi: 10.1038/s41596-018-0119-1 |
| 15 |
Molly K , Woestyne MV , Verstraete W . Development of a 5-step multichamber reactor as a simulation of the human intestinal microbial ecosystem[J]. Appl Microbiol Biot, 1993, 39 (2): 254- 258.
doi: 10.1007/BF00228615 |
| 16 |
杨立娜, 黄靖航, 赵亚凡, 等. 胃肠道体外模拟系统在调控肠道菌群研究中的应用进展[J]. 渤海大学学报(自然科学版), 2018, 39 (4): 320- 329.
doi: 10.3969/j.issn.1673-0569.2018.04.007 |
| 17 |
Laird BD , van de Wiele TR , Corriveau MC , et al. Gastrointestinal microbes increase arsenic bioaccessibility of ingested mine tai-lings using the simulator of the human intestinal microbial ecosystem[J]. Environ Sci Technol, 2007, 41 (15): 5542- 5547.
doi: 10.1021/es062410e |
| 18 | 叶峰, 王晓艳. 粪菌保存液及其保存粪菌的方法, CN105385599A[P/OL]. (2016-03-09)[2022-02-16]. https://pss-system.cponline.cnipa.gov.cn/documents/detail?prevPageTit=chagngui. |
| 19 |
Schiller C , Frohlich CP , Giessmann T , et al. Intestinal fluid vo-lumes and transit of dosage forms as assessed by magnetic resonance imaging[J]. Aliment Pharm Ther, 2005, 22 (10): 971- 979.
doi: 10.1111/j.1365-2036.2005.02683.x |
| 20 |
Khan ST , Saleem S , Ahamed M , et al. Survival of probiotic bacteria in the presence of food grade nanoparticles from chocolates: An in vitro and in vivo study[J]. Appl Microbiol Biot, 2019, 103 (16): 6689- 6700.
doi: 10.1007/s00253-019-09918-5 |
| 21 | Baranowska-Wójcik E, Szwajgier D, Gustaw K. Effect of TiO2 on selected pathogenic and opportunistic intestinal bacteria[J/OL]. Biol Trace Elem Res, (2021-07-23)[2021-12-08]. doi: 10.1007/s12011-021-02843-7. |
| 22 |
Lucas-González R , Viuda-Martos M , Pérez-Alvarez JA , et al. In vitro digestion models suitable for foods: Opportunities for new fields of application and challenges[J]. Food Research International, 2018, 107, 423- 436.
doi: 10.1016/j.foodres.2018.02.055 |
| 23 | Dudefoi W , Moniz K , Allen-Vercoe E , et al. Impact of food grade and nano-TiO2 particles on a human intestinal community[J]. Food Chem Toxicol, 2017, 106 (Pt A): 242- 249. |
| 24 |
Gomaa EZ . Human gut microbiota/microbiome in health and di-seases: A review[J]. Antonie van Leeuwenhoek, 2020, 113 (12): 2019- 2040.
doi: 10.1007/s10482-020-01474-7 |
| 25 |
Nogueira CM , de Azevedo WM , Dagli ML , et al. Titanium dio-xide induced inflammation in the small intestine[J]. World J Gastroenterol, 2012, 18 (34): 4729- 4735.
doi: 10.3748/wjg.v18.i34.4729 |
| 26 | 刘静. 纳米TiO2对肠上皮紧密连接蛋白Occludin和ZO-1的表达及相关细胞信号通道的影响[D]. 南京: 东南大学, 2019. |
| 27 | 陈章健, 王云, 贾光. 纳米二氧化钛食品安全性研究进展[J]. 卫生研究, 2015, 44 (6): 1036- 1041. |
| [1] | Dan HAN, Yangjin CIREN, Qiuhong LI, Jun LI. Changes of intestinal microflora in patients with colorectal benign and malignant tumors in high altitude area and comparison with the normal population in low altitude area [J]. Journal of Peking University (Health Sciences), 2025, 57(3): 578-583. |
| [2] | Jia-qi SHI,Ying MA,Yi ZHANG,Zhang-jian CHEN,Guang JIA. Effects of titanium dioxide nanoparticles on circRNA expression profiles in human hepatocellular carcinoma cells HepG2 [J]. Journal of Peking University (Health Sciences), 2023, 55(3): 392-399. |
| [3] | Wen-han BAO,Wen TANG. Changes of gut microflora in newly diagnosed IgA nephropathy patients and its correlation with clinical risk factors [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 124-132. |
| [4] | WANG Zi-jing,LI Zai-ling. Characteristics of gastric microbiota in children with Helicobacter pylori infection family history [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1115-1121. |
| [5] | Di ZHOU,Zhang-jian CHEN,Gui-ping HU,Teng-long YAN,Chang-mao LONG,Hui-min FENG,Guang JIA. Influence of oxidative/antioxidative biomarkers and inflammatory cytokines on rats after sub-acute orally administration of titanium dioxide nanoparticles [J]. Journal of Peking University (Health Sciences), 2020, 52(5): 821-827. |
| [6] | Shuo HAN,Zhang-jian CHEN,Di ZHOU,Pai ZHENG,Jia-he ZHANG,Guang JIA. Effects of titanium dioxide nanoparticles on fecal metabolome in rats after oral administration for 90 days [J]. Journal of Peking University (Health Sciences), 2020, 52(3): 457-463. |
| [7] | Zhang-jian CHEN,Shuo HAN,Pai ZHENG,Shu-pei ZHOU,Guang JIA. Effect of subchronic combined oral exposure of titanium dioxide nanoparticles and glucose on levels of serum folate and vitamin B12 in young SD rats [J]. Journal of Peking University (Health Sciences), 2020, 52(3): 451-456. |
| [8] | DUAN Shu-min, ZHANG Yong-liang, WANG Yun. Effects of titanium dioxide nanoparticles and lipopolysaccharide on antioxidant function of liver tissues in mice [J]. Journal of Peking University(Health Sciences), 2018, 50(3): 395-400. |
| [9] | WANG Huan, QIN Xiao-ya, LI Zi-yuan, ZHENG Zhuo-zhao, FAN Tian-yuan. Preparation and characterization of citric acid-modified superparamagnetic iron oxide nanoparticles [J]. Journal of Peking University(Health Sciences), 2018, 50(2): 340-346. |
| [10] | ZHANG Yong-liang, CHEN Zhang-jian, CHEN Shi, ZHUO lin, JIA Guang, WANG Yun. Effect of nano-TiO2 on intestinal glucose absorption in young rat on the everted gut sac model [J]. Journal of Peking University(Health Sciences), 2017, 49(3): 376-382. |
| [11] | YANG Di, XU Jun-hui, DENG Fu-rong△, GUO Xin-biao . Effects of silver nanoparticle on hemichannel activation and anti-proliferation in HaCaT cells [J]. Journal of Peking University(Health Sciences), 2017, 49(3): 371-375. |
| [12] | GENG Liang, FAN Jing, GAO Qi-long, YU Jing, HUA Bao-jin. Preliminary study for the roles and mechanisms of 20(R)-ginsenoside Rg3 and PEG-PLGA-Rg3 nanoparticles in the Lewis lung cancer mice [J]. Journal of Peking University(Health Sciences), 2016, 48(3): 496-501. |
| [13] | WANG Yun, CHEN Zhang-Jian, BA Te, PU Ji, CUI Xiao-Xing, JIA Guang. Effects of TiO2 nanoparticles on antioxidant function and element content of liver and kidney tissues in young and adult rats [J]. Journal of Peking University(Health Sciences), 2014, 46(3): 395-399. |
|
||