Journal of Peking University (Health Sciences) ›› 2023, Vol. 55 ›› Issue (2): 351-356. doi: 10.19723/j.issn.1671-167X.2023.02.022
Previous Articles Next Articles
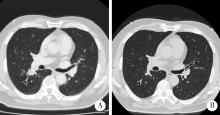
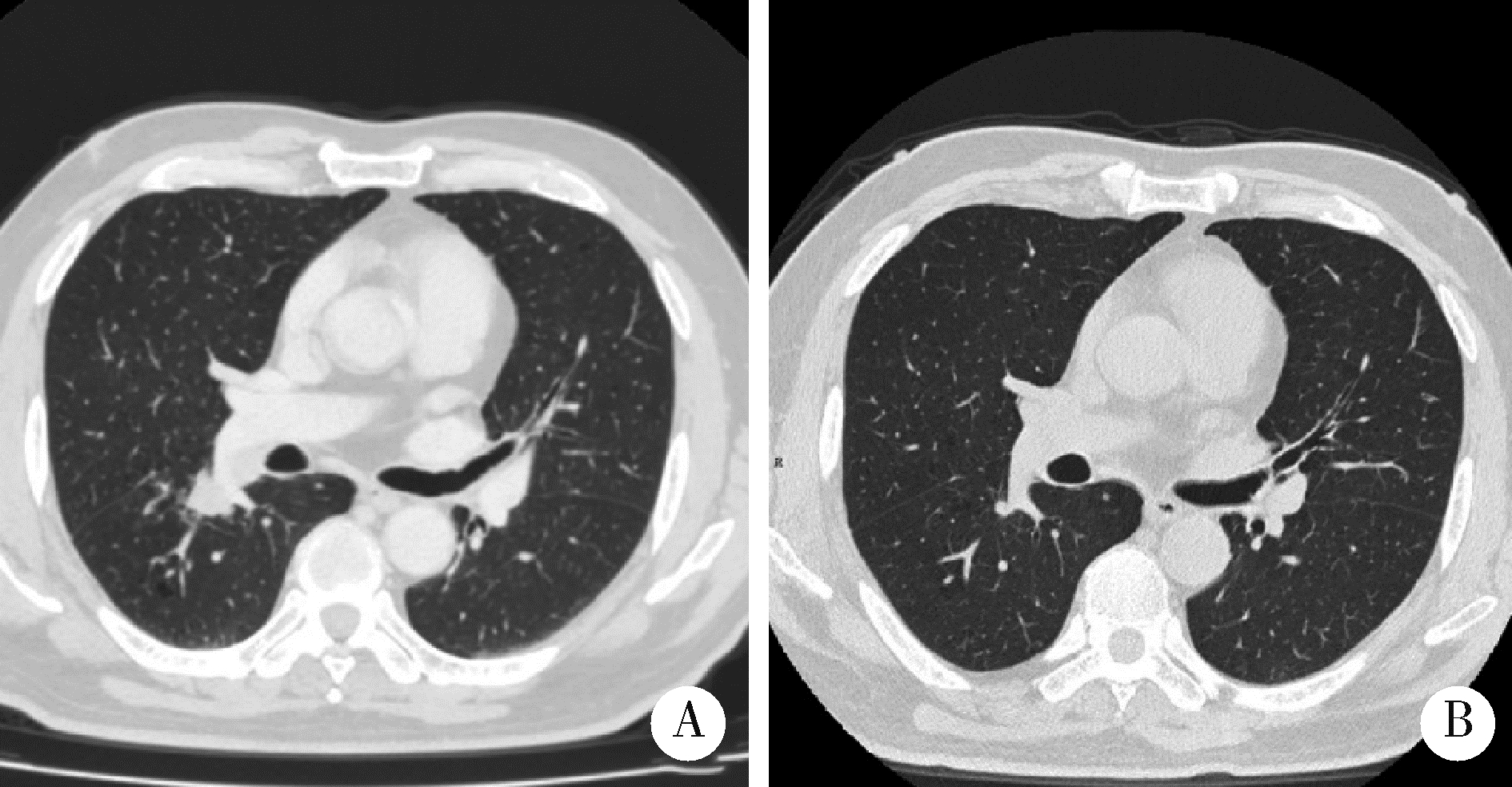
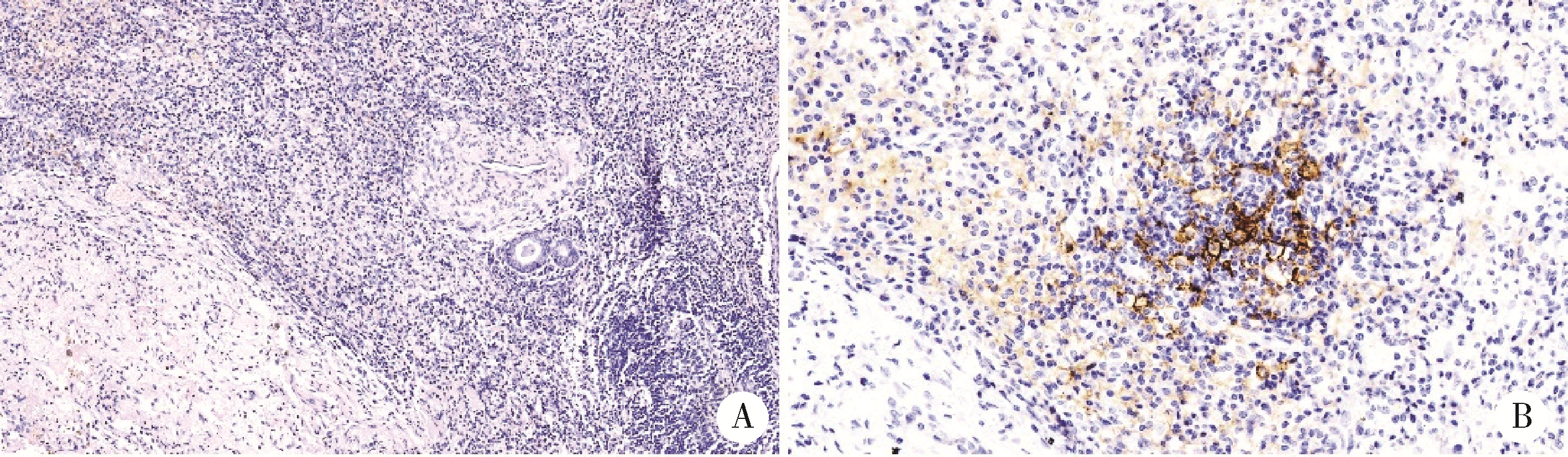
Thoracic SMARCA4-deficient undifferentiated tumor-pathological diagnosis and combined immune checkpoint inhibitor treatment
Yan XIONG1,*( ),Bo ZHANG2,Li-gong NIE3,Shi-kai WU4,Hu ZHAO5,Dong LI1,Ji-ting DI1
),Bo ZHANG2,Li-gong NIE3,Shi-kai WU4,Hu ZHAO5,Dong LI1,Ji-ting DI1
- 1. Department of Pathology, Peking University First Hospital, Beijing 100034, China
2. Department of Pathology, School of Basic Medical Sciences Peking University/Peking University Third Hospital, Beijing 100191, China
3. Department of Respiratory and Critical Care Medicine, Peking University First Hospital, Beijing 100034, China
4. Department of Oncology, Peking University First Hospital, Beijing 100034, China
5. Department of Thoracic Surgery, Peking University First Hospital, Beijing 100034, China
CLC Number:
- R365
| 1 | Travis WD , Bubendorf L , Chung JH , et al. Thoracic SMARCA4-deficient undifferentiated tumor[M]. Lyon Cedex, France: International Agency for Research on Cancer (IARC), 2021: 111- 114. |
| 2 |
Nambirajan A , Jain D . Recent updates in thoracic SMARCA4-deficient undifferentiated tumor[J]. Semin Diagn Pathol, 2021, 38 (5): 83- 89.
doi: 10.1053/j.semdp.2021.06.001 |
| 3 |
Mittal P , Roberts CWM . The SWI/SNF complex in cancer: Biology, biomarkers and therapy[J]. Nat Rev Clin Oncol, 2020, 17 (7): 435- 448.
doi: 10.1038/s41571-020-0357-3 |
| 4 |
Mardinian K , Adashek JJ , Botta GP , et al. SMARCA4: Implications of an altered chromatin-remodeling gene for cancer development and therapy[J]. Mol Cancer Ther, 2021, 20 (12): 2341- 2351.
doi: 10.1158/1535-7163.MCT-21-0433 |
| 5 |
Alessi JV , Ricciuti B , Spurr LF , et al. SMARCA4 and other SWItch/Sucrose NonFermentable family genomic alterations in NSCLC: Clinicopathologic characteristics and outcomes to immune checkpoint inhibition[J]. J Thorac Oncol, 2021, 16 (7): 1176- 1187.
doi: 10.1016/j.jtho.2021.03.024 |
| 6 |
Sasaki M , Ogiwara H . Synthetic lethal therapy based on targeting the vulnerability of SWI/SNF chromatin remodeling complex-deficient cancers[J]. Cancer Sci, 2020, 111 (3): 774- 782.
doi: 10.1111/cas.14311 |
| 7 |
Travis WD , Dacic S , Wistuba I , et al. IASLC multidisciplinary recommendations for pathologic assessment of lung cancer resection specimens after neoadjuvant therapy[J]. J Thorac Oncol, 2020, 15 (5): 709- 740.
doi: 10.1016/j.jtho.2020.01.005 |
| 8 |
Provencio M , Nadal E , Insa A , et al. Neoadjuvant chemotherapy and nivolumab in resectable non small cell lung cancer (NADIM): An open-label, multicentre, single-arm, phase 2 trial[J]. Lancet Oncol, 2020, 21 (11): 1413- 1422.
doi: 10.1016/S1470-2045(20)30453-8 |
| 9 |
Liang W , Cai K , Chen C , et al. Expert consensus on neoadjuvant immunotherapy for non small cell lung cancer[J]. Transl Lung Cancer Res, 2020, 9 (6): 2696- 2715.
doi: 10.21037/tlcr-2020-63 |
| 10 |
Downes MR , Slodkowska E , Katabi N , et al. Inter-and intra-observer agreement of programmed death ligand 1 scoring in head and neck squamous cell carcinoma, urothelial carcinoma and breast carcinoma[J]. Histopathology, 2020, 76 (2): 191- 200.
doi: 10.1111/his.13946 |
| 11 |
Brueckl WM , Ficker JH , Zeitler G . Clinically relevant prognostic and predictive markers for immune-checkpoint-inhibitor (ICI) therapy in non-small cell lung cancer (NSCLC)[J]. BMC Can-cer, 2020, 20 (1): 1185.
doi: 10.1186/s12885-020-07690-8 |
| [1] | Dong LI,Ji-ting DI,Yan XIONG. Consistency comparison of programmed cell death 1-ligand 1 in different immuno-histochemical staining methods [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 339-342. |
| [2] | Sheng-jie LIU,Hui-min HOU,Zheng-tong LV,Xin DING,Lu WANG,Lei ZHANG,Ming LIU. Bipolar androgen therapy followed by immune checkpoint inhibitors in metastatic castration resistant prostate cancer: A report of 4 cases [J]. Journal of Peking University (Health Sciences), 2022, 54(4): 766-769. |
| [3] | Cai-peng QIN,Yu-xuan SONG,Meng-ting DING,Fei WANG,Jia-xing LIN,Wen-bo YANG,Yi-qing DU,Qing LI,Shi-jun LIU,Tao XU. Establishment of a mutation prediction model for evaluating the efficacy of immunotherapy in renal carcinoma [J]. Journal of Peking University (Health Sciences), 2022, 54(4): 663-668. |
| [4] | Yi-cen YING,Qi TANG,Kai-wei YANG,Yue MI,Yu FAN,Wei YU,Yi SONG,Zhi-song HE,Li-qun ZHOU,Xue-song LI. Clinical features of immune checkpoint inhibitor-related myositis in patients with urological cancer [J]. Journal of Peking University (Health Sciences), 2022, 54(4): 644-651. |
| [5] | GU Yang-chun,LIU Ying,XIE Chao,CAO Bao-shan. Pituitary immune-related adverse events induced by programmed cell death protein 1 inhibitors in advanced lung cancer patients: A report of 3 cases [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 369-375. |
| [6] | Wei ZHANG,Ying-jiang YE,Xian-wen REN,Jing HUANG,Zhan-long SHEN. Detection of preoperative chemoradiotherapy sensitivity molecular characteristics of rectal cancer by transcriptome second generation sequencing [J]. Journal of Peking University(Health Sciences), 2019, 51(3): 542-547. |
| [7] | LI Zhi-Hong, LIU Dan, HE Zi-Jing, FAN Zhi-Yi. Influence of dexamethasone on the incidence of postoperative nausea and vomiting in breast cancer patients with neoadjuvant chemotherapy [J]. Journal of Peking University(Health Sciences), 2015, 47(4): 685-689. |
|
||