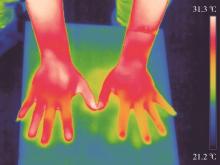
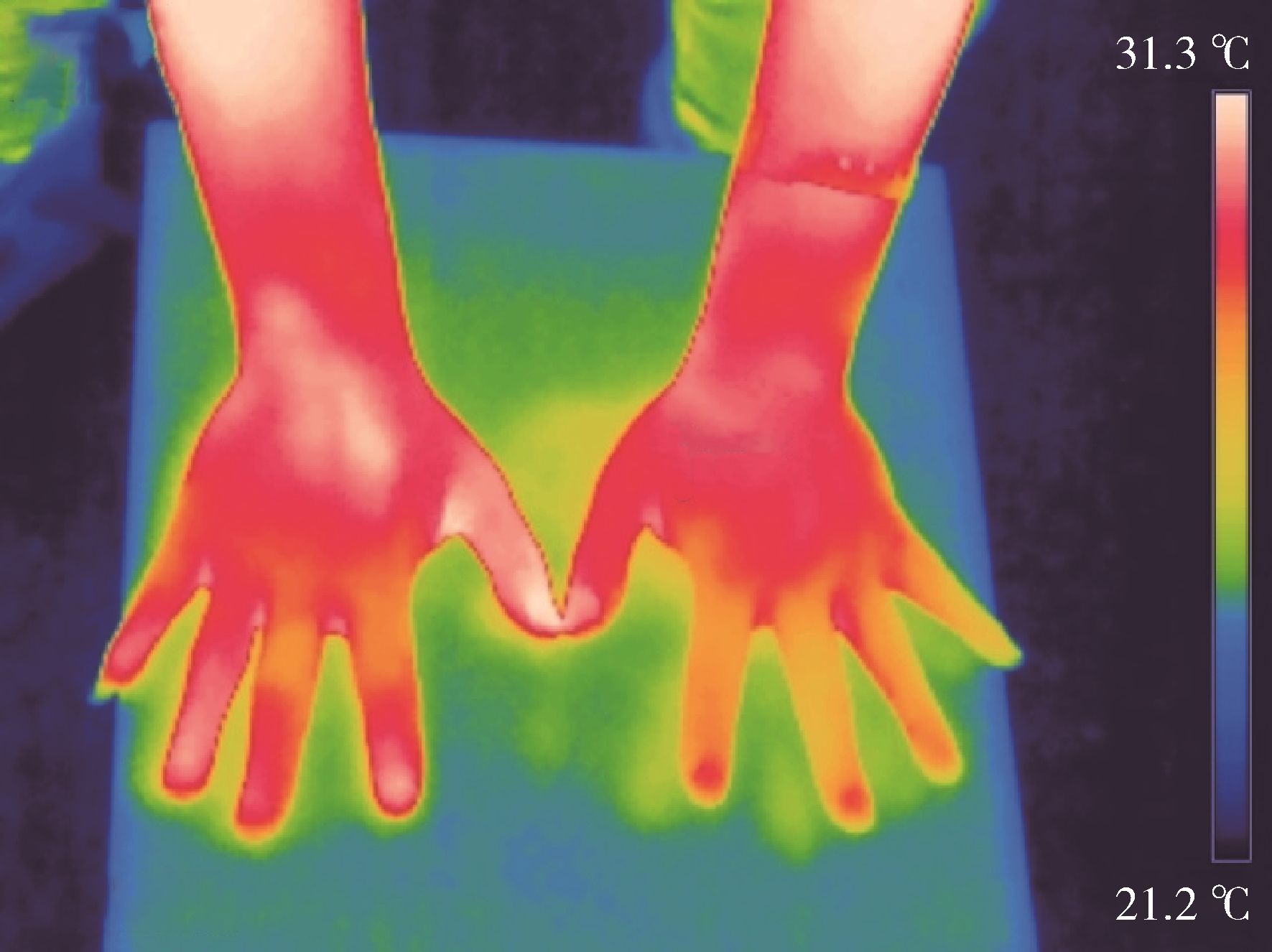
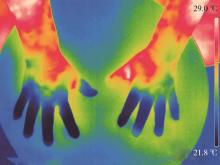
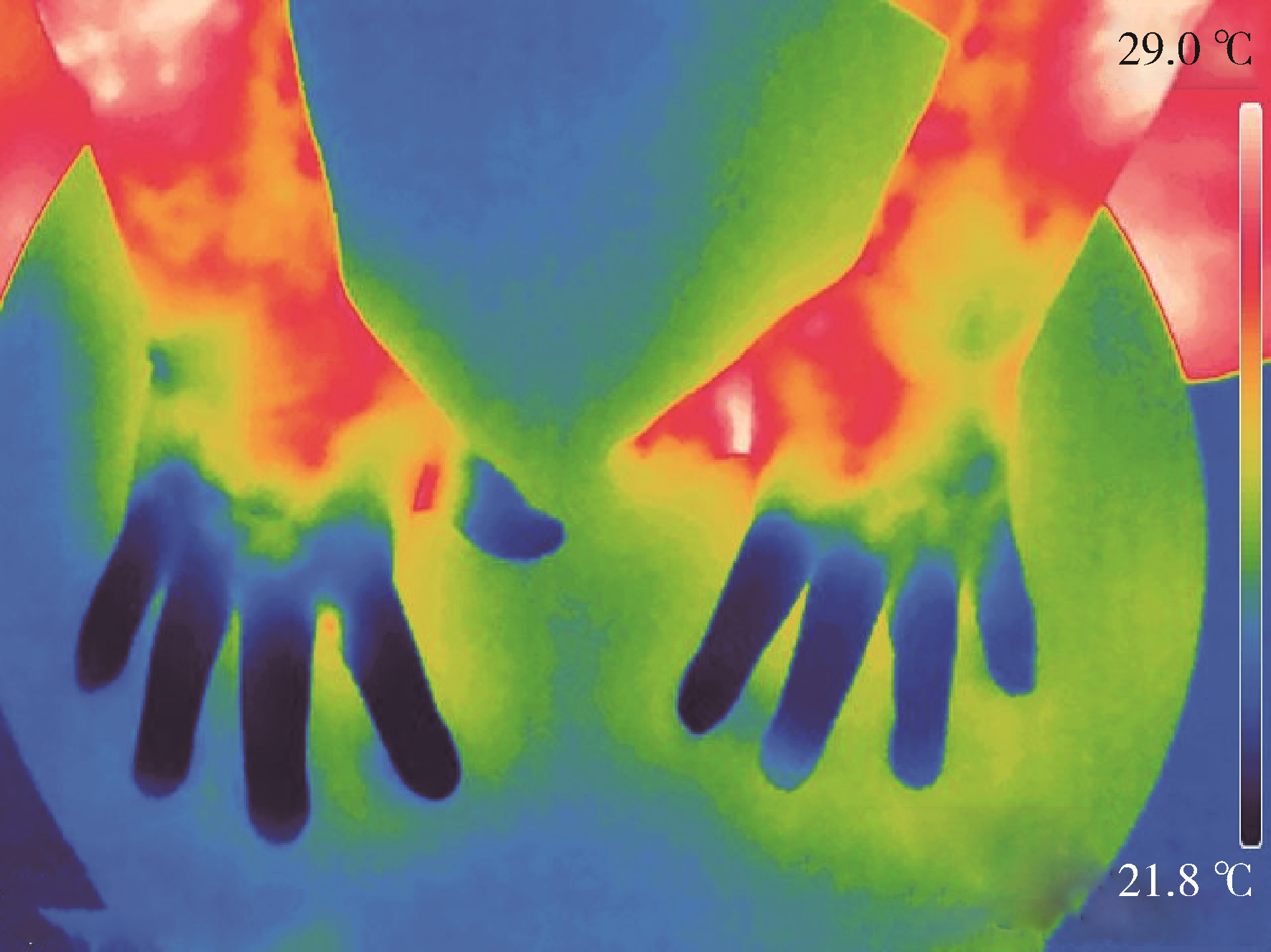
Journal of Peking University (Health Sciences) ›› 2024, Vol. 56 ›› Issue (6): 1132-1136. doi: 10.19723/j.issn.1671-167X.2024.06.031
Previous Articles Next Articles
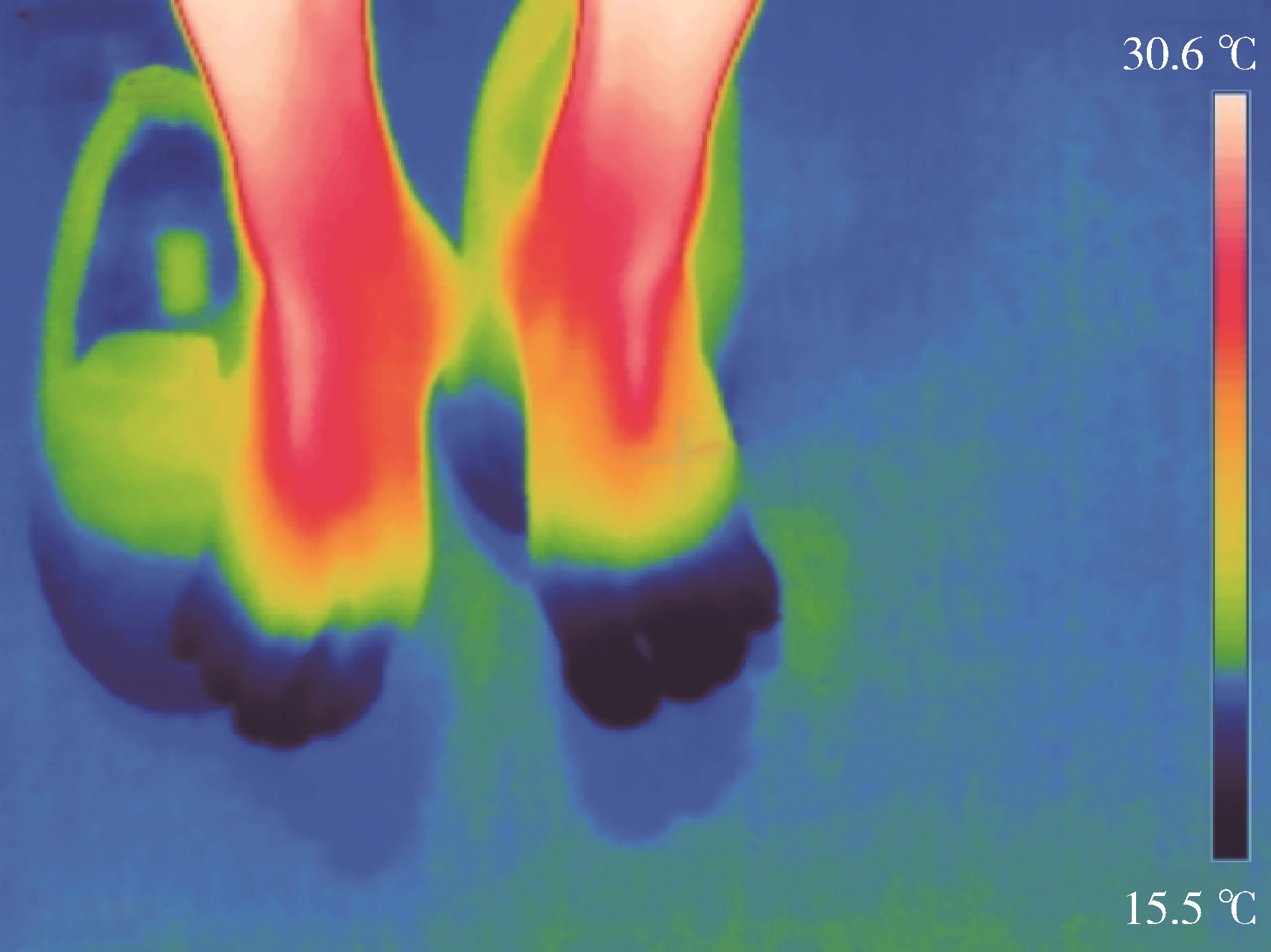
Application and prospects of infrared thermography in rheumatic diseases
Wenxin CAI1, Qiongying YANG2, Dan HAN3, Zhe CHEN1, Yongjing CHENG1,*( )
)
- 1. Department of Rheumatology and Immunology, Beijing Hospital; National Center of Gerontology; Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing 100730, China
2. Department of Integrative Chinese and Western Medicine, Kunming Second People' s Hospital, Kunming 650000, Chian
3. Department of Rheumatology and Immunology, Zhengzhou People' s Hospital, Zhengzhou 450003, China
CLC Number:
- R593.2
| 1 |
Brenner M , Braun C , Oster M , et al. Thermal signature analysis as a novel method for evaluating inflammatory arthritis activity[J]. Ann Rheum dis, 2006, 65 (3): 306- 311.
doi: 10.1136/ard.2004.035246 |
| 2 |
Salisbury RS , Parr G , de Silva M , et al. Heat distribution over normal and abnormal joints: Thermal pattern and quantification[J]. Ann Rheum Dis, 1983, 42 (5): 494- 499.
doi: 10.1136/ard.42.5.494 |
| 3 |
Collins AJ , Ring EF , Cosh JA , et al. Quantitation of thermography in arthritis using multi-isothermal analysis. Ⅰ. The thermographic index[J]. Ann Rheum Dis, 1974, 33 (2): 113- 115.
doi: 10.1136/ard.33.2.113 |
| 4 |
Collins AJ , Cosh JA . Temperature and biochemical studies of joint inflammation. A preliminary investigation[J]. Ann Rheum Dis, 1970, 29 (4): 386- 392.
doi: 10.1136/ard.29.4.386 |
| 5 |
Wevers-de Boer KVC , Heimans L , Huizinga TWJ , et al. Drug therapy in undifferentiated arthritis: A systematic literature review[J]. Ann Rheum Dis, 2013, 72 (9): 1436- 1444.
doi: 10.1136/annrheumdis-2012-203165 |
| 6 |
Gatt A , Mercieca C , Borg A , et al. A comparison of thermogra-phic characteristics of the hands and wrists of rheumatoid arthritis patients and healthy controls[J]. Sci Rep, 2019, 9 (1): 17204.
doi: 10.1038/s41598-019-53598-0 |
| 7 |
Nosrati Z , Bergamo M , Rodríguez-Rodríguez C , et al. Refinement and validation of infrared thermal imaging (IRT): A non-invasive technique to measure disease activity in a mouse model of rheumatoid arthritis[J]. Arthritis Res Ther, 2020, 22 (1): 281.
doi: 10.1186/s13075-020-02367-w |
| 8 |
Varjú G , Pieper CF , Renner JB , et al. Assessment of hand osteoarthritis: Correlation between thermographic and radiographic methods[J]. Rheumatology (Oxford), 2004, 43 (7): 915- 919.
doi: 10.1093/rheumatology/keh204 |
| 9 | 吴穗岚. 基于红外热图特征提取的膝关节炎诊断系统研究[D]. 杭州: 中国计量大学, 2019. |
| 10 |
Petrigna L , Amato A , Roggio F , et al. Thermal threshold for knee osteoarthritis people evaluated with infrared thermography: A scoping review[J]. J Therm Biol, 2024, 123, 103932.
doi: 10.1016/j.jtherbio.2024.103932 |
| 11 | 杨瑞宇, 李鹏, 王玮玉, 等. 基于红外热成像技术的急性痛风性关节炎治疗效果评价研究[J]. 红外, 2021, 42 (6): 45- 48. |
| 12 | 王建军, 程红霞. 红外热成像对口服乌鸡白凤丸联合苯溴马隆片治疗急性痛风性关节炎的疗效评价[J]. 青海医药杂志, 2019, 49 (6): 49- 51. |
| 13 | Vasdev V , Singh R , Aggarwal V , et al. Thermal imaging in rheumatoid arthritis knee joints and its correlation with power Doppler ultrasound[J]. Med J Armed Forces India, 2023, 79 (Suppl 1): S189- S195. |
| 14 | Triantafyllias K , Clasen M , de Blasi M , et al. Performance of a novel high-resolution infrared thermography marker in detecting and assessing joint inflammation: A comparison with joint ultrasound[J]. Clin Exp Rheumatol, 2024, 42 (9): 1802- 1811. |
| 15 |
Morales-Ivorra I , Narváez J , Gómez-Vaquero C , et al. A thermographic disease activity index for remote assessment of rheumatoid arthritis[J]. RMD Open, 2022, 8 (2): e002615.
doi: 10.1136/rmdopen-2022-002615 |
| 16 |
Morales-Ivorra I , Narváez J , Gómez-Vaquero C , et al. Assessment of inflammation in patients with rheumatoid arthritis using thermo-graphy and machine learning: A fast and automated technique[J]. RMD Open, 2022, 8 (2): e002458.
doi: 10.1136/rmdopen-2022-002458 |
| 17 |
Tan YK , Hong C , Li H , et al. A novel use of combined thermal and ultrasound imaging in detecting joint inflammation in rheumatoid arthritis[J]. Eur J Radiol, 2021, 134, 109421.
doi: 10.1016/j.ejrad.2020.109421 |
| 18 |
Tan YK , Hong C , Li H , et al. Receiver operating characteristic analysis using a novel combined thermal and ultrasound imaging for assessment of disease activity in rheumatoid arthritis[J]. Sci Rep, 2022, 12 (1): 22115.
doi: 10.1038/s41598-022-26728-4 |
| 19 |
Lim MJ , Kwon SR , Jung KH , et al. Digital thermography of the fingers and toes in Raynaud' s phenomenon[J]. J Korean Med Sci, 2014, 29 (4): 502- 506.
doi: 10.3346/jkms.2014.29.4.502 |
| 20 | Cherkas LF , Carter L , Spector TD , et al. Use of thermographic criteria to identify Raynaud' s phenomenon in a population setting[J]. J Rheumatol, 2003, 30 (4): 720- 722. |
| 21 |
Schuhfried O , Vacariu G , Lang T , et al. Thermographic parameters in the diagnosis of secondary Raynaud' s phenomenon[J]. Arch Phys Med Rehabil, 2000, 81 (4): 495- 499.
doi: 10.1053/mr.2000.4870 |
| 22 |
Anderson ME , Moore TL , Lunt M , et al. The 'distal-dorsal difference': A thermographic parameter by which to differentiate between primary and secondary Raynaud' s phenomenon[J]. Rheumatology (Oxford), 2007, 46 (3): 533- 538.
doi: 10.1093/rheumatology/kel330 |
| 23 |
Panasiti MS , Ponsi G , Monachesi B , et al. Cognitive load and emotional processing in psoriasis: A thermal imaging study[J]. Exp Brain Res, 2019, 237 (1): 211- 222.
doi: 10.1007/s00221-018-5416-y |
| 24 |
Vergilio MM , Gomes G , Aiello LM , et al. Evaluation of skin using infrared thermal imaging for dermatology and aesthetic applications[J]. J Cosmet Dermatol, 2022, 21 (3): 895- 904.
doi: 10.1111/jocd.14748 |
| 25 |
Pain CE , Murray A , Dinsdale G , et al. Non-invasive imaging and clinical skin scores in juvenile localized scleroderma[J]. Rheumatology (Oxford), 2024, 63 (5): 1332- 1340.
doi: 10.1093/rheumatology/kead380 |
| 26 | 颜志芳. 消银软膏治疗银屑病血热证的疗效评价[D]. 北京: 中国中医科学院, 2006. |
| 27 | Castillo-Martinez C , Valdes-Rodriguez R , Kolosovas-Machuca ES , et al. Use of digital infrared imaging in the assessment of childhood psoriasis[J]. Skin Res Technol, 2013, 19 (1): e549- e551. |
| [1] | Peiwen JIA, Ying YANG, Yaowei ZOU, Zhiming OUYANG, Jianzi LIN, Jianda MA, Kuimin YANG, Lie DAI. Clinical characteristics of overlapping syndromes of low muscle mass in patients with rheumatoid arthritis and their impact on physical function [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 1009-1016. |
| [2] | Doudou MA, Zhemin LU, Qian GUO, Sha ZHU, Jin GU, Yan DING, Lianjie SHI. Successful treatment of rheumatoid arthritis complicated with myasthenia gravis with low-dose rituximab: A case report [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 1110-1114. |
| [3] | Rui YAN, Dan KE, Yan ZHANG, Li LI, Huanran SU, Wei CHEN, Mingxia SUN, Xiaomin LIU, Liang LUO. Diagnostic significance of serum chemokine CXCL-10 and Krebs von den lungen-6 level in patients with rheumatoid arthritis associated interstitial lung disease [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 956-962. |
| [4] | Liang ZHAO, Chenglong SHI, Ke MA, Jing ZHAO, Xiao WANG, Xiaoyan XING, Wanxing MO, Yirui LIAN, Chao GAO, Yuhui LI. Immunological characteristics of patients with anti-synthetase syndrome overlap with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 972-979. |
| [5] | Jiayu ZHAI, Jinxia ZHAO, Zhuo AN, Rui LIU. Assessment of residual symptoms in patients with axial spondyloarthritis with low disease activity and analysis of its related factors [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 987-993. |
| [6] | Yijun HAN, Xiaoli CHEN, Changhong LI, Jinxia ZHAO. Application status of methotrexate in patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 994-1000. |
| [7] | Dongwu LIU, Jie CHEN, Mingli GAO, Jing YU. Rheumatoid arthritis with Castleman-like histopathology in lymph nodes: A case report [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 928-931. |
| [8] | Zhengfang LI,Cainan LUO,Lijun WU,Xue WU,Xinyan MENG,Xiaomei CHEN,Yamei SHI,Yan ZHONG. Application value of anti-carbamylated protein antibody in the diagnosis of rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 729-734. |
| [9] | Huina HUANG,Jing ZHAO,Xiangge ZHAO,Ziran BAI,Xia LI,Guan WANG. Regulatory effect of lactate on peripheral blood CD4+ T cell subsets in patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 519-525. |
| [10] | Xiaofei TANG,Yonghong LI,Qiuling DING,Zhuo SUN,Yang ZHANG,Yumei WANG,Meiyi TIAN,Jian LIU. Incidence and risk factors of deep vein thrombosis in patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 279-283. |
| [11] | Hongguang LI,Weihua HAN,Xun WU,Jiling FENG,Gang LI,Juanhong MENG. Preliminarily study of arthrocentesis combined with liquid phase concentrated growth factor injection in the treatment of unilateral temporomandibular joint osteoarthritis [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 338-344. |
| [12] | Xue ZOU,Xiao-juan BAI,Li-qing ZHANG. Effectiveness of tofacitinib combined with iguratimod in the treatment of difficult-to-treat moderate-to-severe rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1013-1021. |
| [13] | Qi WU,Yue-ming CAI,Juan HE,Wen-di HUANG,Qing-wen WANG. Correlation between dyslipidemia and rheumatoid arthritis associated interstitial lung disease [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 982-992. |
| [14] | Jing-feng ZHANG,Yin-ji JIN,Hui WEI,Zhong-qiang YAO,Jin-xia ZHAO. Correlation analysis between body mass index and clinical characteristics of rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 993-999. |
| [15] | Qing PENG,Jia-jun LIU,Yan LIU,Hua SHANG,Guo TANG,Ya-xin HAN,Li LONG. Application of Padua prediction score and serum albumin level in evaluating venous thromboembolism in rheumatic inpatients [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 625-630. |
|
||