吴茱萸(Evodiae fructus)始载于《神农本草经》,为临床常用的传统中药,具有散寒止痛,降逆止呕,助阳止泻的功效。吴茱萸有小毒[1],临床上常因服用生品吴茱萸、未制透的吴茱萸或超剂量服用而中毒,研究表明肝脏是吴茱萸的主要毒性靶器官[2,3],但尚未阐明其毒性物质基础及毒性机制。吴茱萸碱(evodiamine,EVO)属色胺吲哚类生物碱,是吴茱萸主要生物碱成分之一,具有抗肿瘤[4]、抗炎[5]、抑菌[6]、镇痛[7]、神经保护[8]等药理作用。EVO可能是吴茱萸肝毒性的物质基础,其可降低人正常肝细胞L-02存活率,升高谷丙转氨酶(alanine transaminase,ALT)、谷草转氨酶(aspartate amino-transferase,AST)、乳酸脱氢酶(lactate dehydrogenase,LDH)和碱性磷酸酶(alkaline phosphatase,ALP)活性[9],但其肝毒性机制尚不明确。动物实验发现吴茱萸肝毒性机制与氧化损伤相关[10],可导致ALP活性和总胆红素(total bilirubin,TBIL)含量升高[3],并促使肝细胞凋亡[11]。HepG2细胞具有许多肝脏特异性相关功能,是目前用于评估肝毒性的人源细胞模型之一,广泛应用于药物肝毒性及分子机制研究[12],因此,本研究用HepG2细胞研究EVO的肝细胞毒性作用特点,从脂质过氧化损伤、凋亡和胆汁淤积三方面初步探讨其毒性机制。
1 资料与方法
1.1 细胞系
HepG2细胞由北京大学公共卫生学院毒理学系赵鹏老师惠赠。
1.2 药物及主要试剂
EVO由北京大学药学院天然药物学系杨秀伟教授提供,纯度≥98%。细胞增殖及毒性检测(cell counting kit-8,CCK-8)试剂盒、超氧化物歧化酶(superoxide dismutase,SOD)检测试剂盒、丙二醛(malondialdehyde,MDA)检测试剂盒和异硫氰酸荧光素标记的膜联蛋白/碘化丙啶(annexin V-fluorescein isothiocyanate/propidium iodide,Annexin V-FITC/PI)细胞凋亡检测试剂盒购自上海碧云天生物技术有限公司,ALT、AST、LDH、ALP和TBIL检测试剂盒购自南京建成生物工程有限公司,线粒体膜电位荧光探针(superior alternative to JC-1,JC-10)检测试剂盒购自北京索莱宝科技有限公司,小鼠抗人β-actin抗体(TA-09)、辣根酶标记山羊抗兔IgG(ZB-5301)和辣根酶标记山羊抗小鼠IgG(ZB-2305)购自北京中杉金桥生物技术有限公司,兔抗人caspase-9(ab25758)、caspase-3(ab32351)、胆盐输出泵(bile salt export pump,BSEP,ab155421)和多耐药相关蛋白2(multidrug resistance-associated protein 2,MRP2,ab172630)抗体购自英国Abcam公司。
1.3 主要仪器
HERAcell 150i CO2培养箱购自美国Thermo Sscientific公司,FLUOstar Omega多功能酶标仪购自德国BMG公司,CytoFLEX流式细胞仪购自美国Beckman Coulter公司,Mini-PROTEAN Tetra Cell小垂直板电泳转印系统购自美国Bio-Rad公司。
1.4 实验方法
1.4.1 细胞培养 将HepG2细胞培养在有10%(体积分数)胎牛血清、100 U/mL青霉素和0.1 g/L链霉素的达尔伯克改良伊格尔培养基(Dulbecco’s modified Eagle’s medium,DMEM)中,置于37 ℃、95%湿度、5%(体积分数)CO2培养箱,2~3 d传代一次。
1.4.2 CCK-8法检测细胞存活率 将对数生长期的HepG2细胞(1×105/mL)接种于96孔板,每孔100 μL。24 h细胞贴壁后弃上清液,磷酸盐缓冲液(phosphate buffer saline,PBS)洗涤一次,加入含EVO(0、0.04、0.2、1、5和25 μmol/L)无血清培养基,分别处理24、48和72 h,0 μmol/L组作为对照组,其他剂量组作为EVO实验组。处理结束后向每孔加入CCK-8溶液10 μL,37 ℃水浴30 min,用多功能酶标仪测定450 nm处光密度值,计算细胞存活率和半数抑制浓度(half maximal inhibitory concentration,IC50)。
1.4.3 细胞培养上清液中ALT、AST、LDH、ALP活性和TBIL含量检测 将对数生长期的HepG2细胞(2×105/mL)接种于24孔板,每孔0.5 mL。24 h细胞贴壁后弃上清液,PBS洗涤一次,加入含EVO(0、0.2、1和5 μmol/L)无血清培养基处理48 h。吸取细胞培养液,600×g离心5 min取上清液,按照ALT、AST、LDH、ALP和TBIL试剂盒说明书进行操作,用多功能酶标仪分别检测相应的光密度值,计算ALT、AST、LDH、ALP活性和TBIL含量。
1.4.4 SOD活性和MDA含量检测 将对数生长期的HepG2细胞(2×105/mL)接种于6孔板,每孔2 mL。EVO处理HepG2细胞方法同第1.4.3小节。用裂解液裂解细胞后,于4 ℃、14 000×g离心5 min,取上清液并用二喹啉甲酸法(bicinchoninic acid assay,BCA)测定其总蛋白浓度,按照SOD和MDA试剂盒说明书分别测定SOD活性和MDA含量。
1.4.5 分子对接 用SYBYL-X 2.0软件,将EVO与凋亡相关蛋白caspase-9和caspase-3、自噬相关蛋白选择性自噬接头蛋白(sequestosome-1,SQSTM1/p62)、Beclin-1和微管相关蛋白轻链3(microtubule-associated protein light chain-3,LC3)和铁死亡相关蛋白谷胱甘肽过氧化酶4(glutathione peroxidase 4,Gpx4)进行分子对接。蛋白质三维结构从蛋白质数据库(protein data bank,PDB)获取。对接前先对蛋白进行处理,获得能量最小化的稳定结构。对EVO进行能量最小化计算。选择高精度模式,提取配体分子,生成蛋白结合活性口袋,进行半柔性分子对接[13]。对接结果采用总得分值进行评价,总得分值大于5即认为EVO可与靶蛋白结合,且得分越高代表结合越强。采用PyMOL2.7 和LigPLOT软件将EVO与靶蛋白对接结果进行可视化。
1.4.6 线粒体膜电位检测 HepG2细胞培养以及EVO处理细胞方法同第1.4.4小节。胰酶消化后收集细胞,加入JC-10染色工作液,置于细胞培养箱中37 ℃避光孵育20 min,用JC-10染色缓冲液(1×)洗涤2次,经300目细胞筛制成单细胞悬液,流式细胞仪检测红、绿荧光强度,计算红、绿荧光相对比例。
1.4.7 Annexin V-FITC/PI探针检测细胞凋亡 HepG2细胞培养以及EVO处理细胞方法同第1.4.4小节。收集细胞培养液,PBS洗涤一次,用不含Ca2+、Mg2+的乙二胺四乙酸(ethylenediaminetetraacetic acid,EDTA)胰酶消化后收集细胞,800×g离心5 min,弃上清液,PBS重悬细胞并计数。取(5~10)×104细胞,600×g离心5 min后弃上清液,加入195 μL Annexin V-FITC结合液,再分别加入0.2 μL Annexin V-FITC和2.5 μL PI染色液,室温避光孵育20 min,流式细胞仪检测Annexin V-FITC的绿色荧光和PI的红色荧光,计算细胞凋亡率。
1.4.8 Western blot检测凋亡蛋白及胆汁酸转运体蛋白表达量 将对数生长期的3.5×106个HepG2细胞接种于25 cm2培养瓶,EVO处理细胞方法同第1.4.4小节。提取总蛋白,并用BCA法测定其浓度,加入5×蛋白上样缓冲液后,沸水浴7 min使蛋白变性。20 μg等量蛋白上样,40 mA、100 min进行十二烷基硫酸钠聚丙烯酰胺凝胶电泳(sodium dodecyl sulfate polyacrylamide gel electrophoresis,SDS-PAGE),随后使用聚偏二氟乙烯(polyvinylidene fluoride,PVDF)印迹膜300 mA电转90 min,6%(质量分数)脱脂奶粉溶液封闭2 h,含吐温20的Tris缓冲盐溶液(tris-buffered saline with tween-20,TBST)清洗三次,每次10 min,4 ℃孵育一抗过夜。TBST清洗三次,每次10 min,室温孵育二抗1 h。TBST清洗三次,每次10 min,加极超敏化学发光试剂曝光成像。
1.5 统计学分析
数据分析采用SPSS 24.0软件,实验结果数据以$\overline{x}±s$表示,多组间差异采用单因素方差(one-way analysis of variance,ANOVA)分析,P<0.05为差异具有统计学意义。
2 结果
2.1 EVO对HepG2细胞存活率的影响
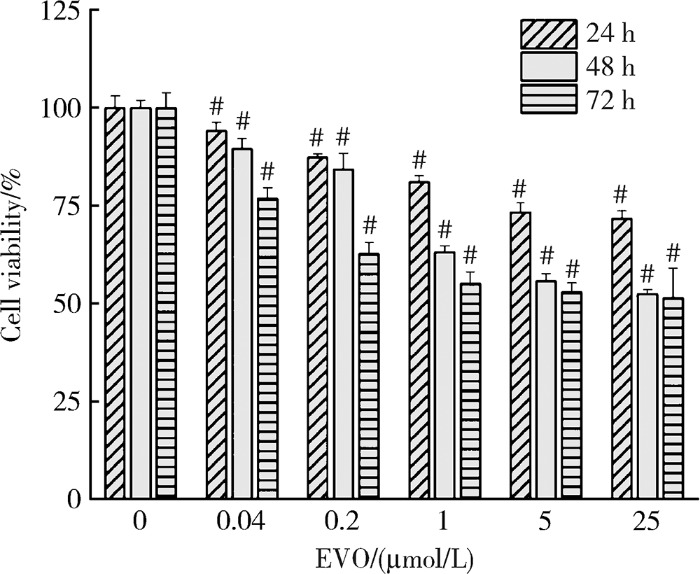
如图1所示,与对照组相比,EVO实验组HepG2细胞存活率均降低,且呈剂量和时间依赖性,其中0.2、1和5 μmol/L EVO处理HepG2细胞24 h,细胞存活率分别为87.3%、81.1%和73.3%;处理48 h,细胞存活率分别为84.3%、63.1%和55.8%;处理72 h,细胞存活率分别为62.7%,55.1%和52.9%。EVO处理HepG2细胞24、48和72 h的IC50分别为85.3、6.6和4.7 μmol/L。
图1
图1
不同浓度和时间下的EVO对HepG2细胞存活率的影响
Figure 1
Effects of EVO on viability of HepG2 cells at different concentrations and time
$\overline{x}$±s, n=3. # P<0.01, compared with control group at the same time. EVO, evodiamine.
基于EVO处理HepG2细胞的IC50,考虑吴茱萸药材中EVO含量和人体摄入量,选择0.2、1、5 μmol/L为EVO低、中、高剂量处理细胞48 h作为后续实验条件。
2.2 EVO对HepG2细胞肝功能生化指标的影响
如表1所示,与对照组相比,不同浓度EVO处理HepG2细胞48 h后,细胞培养上清液中ALT、AST、LDH、ALP活性和TBIL含量均不同程度升高,且ALT、AST和ALP升高在5 μmol/L EVO组差异具有统计学意义(P<0.01),LDH升高在0.2、1和5 μmol/L EVO组差异均具有统计学意义(P<0.05), TBIL升高在1和5 μmol/L EVO组差异均具有统计学意义(P<0.01)。
表1 EVO处理HepG2细胞48 h对肝功能生化指标的影响($\overline{x}±s$, n=3)
Table 1
| Group | ALT/(U/L) | AST/(U/L) | LDH/(U/L) | ALP/(King’s unit/100 mL) | TBIL/(μmol/L) |
|---|---|---|---|---|---|
| 0 μmol/L | 2.31±0.82 | 5.17±0.69 | 158.04±25.00 | 0.35±0.03 | 4.54±1.45 |
| 0.2 μmol/L | 2.70±1.16 | 5.42±0.35 | 187.80±28.37* | 0.34±0.02 | 6.09±1.66 |
| 1 μmol/L | 2.61±1.17 | 5.43±0.84 | 231.92±19.92# | 0.37±0.02 | 13.23±3.15# |
| 5 μmol/L | 5.82±1.26# | 6.34±0.75# | 242.11±19.72# | 0.39±0.05# | 16.06±3.72# |
* P<0.05, # P<0.01, compared with control group. EVO, evodiamine; ALT, alanine transaminase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; ALP, alkaline phosphatase; TBIL, total bilirubin.
2.3 EVO对SOD活性和MDA含量的影响
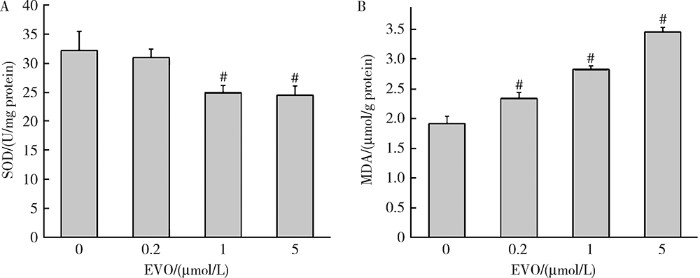
如图2所示,0.2、1和5 μmol/L EVO处理HepG2细胞48 h后,SOD酶活性从对照组的32.17 U/mg蛋白分别降低至30.96、24.95和24.47 U/mg蛋白,且在1和5 μmol/L EVO组差异具有统计学意义(P<0.01)。MDA含量从对照组的1.91 μmol/g蛋白分别升高至2.34、2.83和3.45 μmol/g蛋白,差异均具有统计学意义(P<0.01)。上述结果表明,EVO可导致HepG2细胞脂质过氧化损伤。
图2
图2
EVO对HepG2细胞脂质过氧化损伤的影响
Figure 2
Effects of EVO on lipid peroxidation damage in HepG2 cells
A, SOD activity; B, MDA content. $\overline{x}$±s. n=3. HepG2 cells were treated with EVO for 48h. # P<0.01, compared with control group. EVO, evodiamine; SOD, superoxide dismutase; MDA, malondialdehyde.
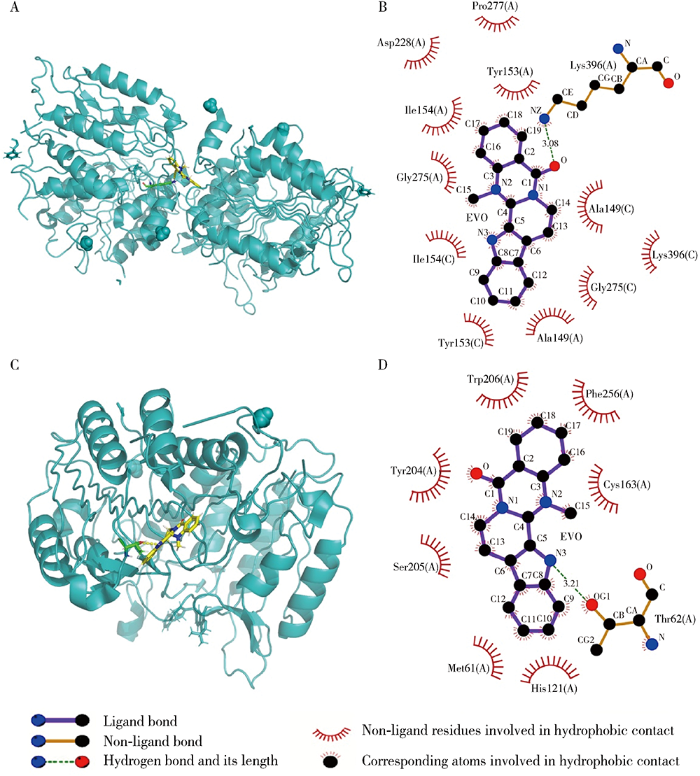
2.4 EVO与凋亡、自噬和铁死亡相关蛋白的对接
表2 EVO与凋亡、自噬和铁死亡相关蛋白对接
Table 2
| Protein | PDB ID | Total score | H-bond number | Residues involved in H-bond formation |
|---|---|---|---|---|
| Caspase-9 | 1JXQ | 6.31 | 1 | A/Lys396 |
| Caspase-3 | 3H0E | 5.13 | 1 | A/Thr62 |
| p62 | 6MJ7 | 3.01 | 0 | |
| Beclin-1 | 5EFM | 2.61 | 0 | |
| LC3 | 3WAM | 3.99 | 0 | |
| Gpx4 | 6HKQ | 4.03 | 1 | A/Lys48 |
EVO, evodiamine; PDB, protein data bank; p62, sequestosome-1; LC3, microtubule-associated protein light chain-3; Gpx4, glutathione peroxidase 4; ID, identity document.
图3
图3
EVO与人源凋亡相关蛋白相互作用
Figure 3
Molecular interaction between EVO and human apoptosis-associated proteins
A, the 3D model of caspase-9; B, the 2D model of caspase-9; C, the 3D model of caspase-3; D, the 2D model of caspase-3. EVO, evodiamine.
2.5 EVO对HepG2细胞线粒体膜电位的影响
如图4所示,0.2、1和5 μmol/L EVO处理HepG2细胞48 h后,与对照组(87.2%)比较,红、绿荧光相对比例分别降低至 71.9%,63.7%和72.6%,且差异均具有统计学意义(P<0.01),说明EVO可导致HepG2细胞线粒体膜电位的下降。
图4
图4
EVO对HepG2细胞线粒体膜电位的影响
Figure 4
Effects of EVO on mitochondrial membrane potential in HepG2 cells
$\overline{x}$±s. n=3. HepG2 cells were treated with EVO for 48 h. # P<0.01, compared with control group. EVO, evodiamine.
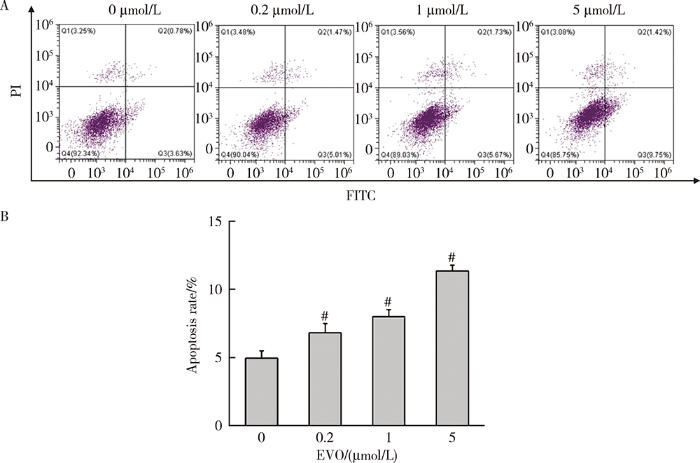
2.6 EVO对HepG2细胞凋亡的影响
如图5所示,0.2、1和5 μmol/L EVO处理HepG2细胞48 h后,与对照组(4.9%)比较,细胞凋亡比例分别升高至6.8%、8.0%和11.3%,差异均具有统计学意义(P<0.01)。
图5
图5
EVO对HepG2细胞凋亡率的影响
Figure 5
Effects of EVO on apoptosis rate in HepG2 cells
A, apoptosis was evaluated by Annexin V-FITC and PI staining; B, quantitative statistical results of the proportion of apoptotic cells. $\overline{x}$±s. n=3. HepG2 cells were treated with EVO for 48h. # P<0.01 compared with control group. FITC, fluorescein isothiocyanate; PI, propidium iodide; EVO, evodiamine.
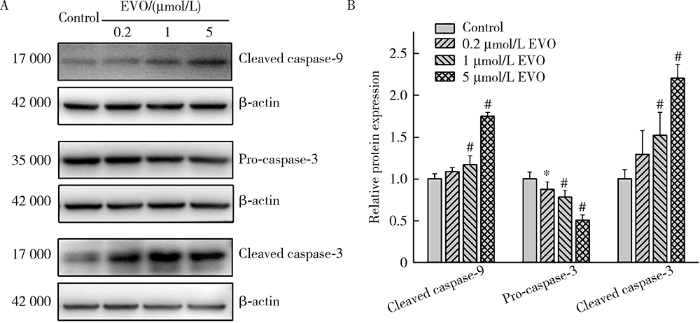
2.7 EVO对HepG2细胞凋亡相关蛋白的影响
如图6所示,EVO处理HepG2细胞48 h后,0.2、1和5 μmol/L EVO组的caspase-9剪切蛋白表达量分别为对照组的1.1、1.2和1.8倍,caspase-3剪切蛋白表达量分别为对照组的1.3、1.5和2.2倍,在1和5 μmol/L EVO组差异均具有统计学意义(P<0.01),caspase-3前体蛋白表达量分别为对照组的87.2%、78.0%和50.4%,差异均具有统计学意义(P<0.05),上述结果表明EVO可引发HepG2细胞凋亡。
图6
图6
EVO对HepG2细胞caspase-9和caspase-3蛋白表达的影响
Figure 6
Effects of EVO on the protein expressions of caspase-9 and caspase-3 in HepG2 cells
A, relative protein expression levels by Western blot; B, semi-quantitative analysis. $\overline{x}$±s. n=3. HepG2 cells were treated with EVO for 48 h. * P<0.05, # P<0.01, compared with control group. EVO, evodiamine.
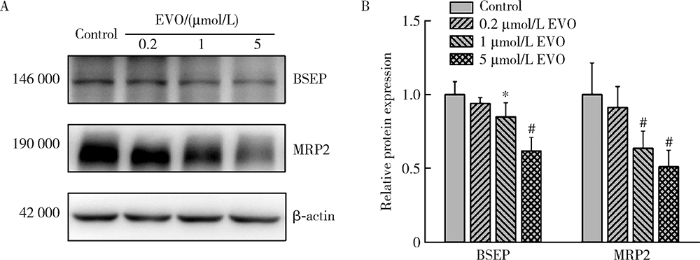
2.8 EVO对BSEP和MRP2蛋白表达的影响
BSEP和MRP2是重要的胆汁酸转运体,如图7所示,0.2、1和5 μmol/L EVO处理HepG2细胞48 h后,BSEP蛋白表达量分别为对照组的94.0%、84.8%和61.8%,MRP2蛋白表达量分别为对照组的91.2%、63.5%和51.1%,在1和5 μmol/L EVO组差异均具有统计学意义(P<0.05),表明EVO可抑制HepG2细胞胆汁酸转运体BSEP和MRP2,使胆汁酸外排受阻从而导致胆汁淤积的发生。
图7
图7
EVO对HepG2细胞BSEP和MRP2蛋白表达的影响
Figure 7
Effects of EVO on the protein expressions of BSEP and MRP2 in HepG2 cells
A, relative protein expression levels by Western blot; B, semi-quantitative analysis. $\overline{x}$±s. n=3. HepG2 cells were treated with EVO for 48 h. * P<0.05, # P<0.01, compared with control group. EVO, evodiamine; BSEP, bile salt export pump; MRP2, multidrug resistance-associated protein 2.
3 讨论
本研究中,CCK-8实验结果显示EVO可降低HepG2细胞存活率,且呈时间和剂量依赖性。ALT主要存在于肝细胞中,具有较好的组织特异性,AST存在于肝脏、肾脏、心脏和骨骼肌中,当肝细胞受损导致细胞膜通透性增加时,ALT和AST会被释放出来,使血清或肝细胞培养液中的酶活力增高,通常结合这两种酶来判断肝损伤情况。LDH是细胞质内稳定表达的酶,可以通过测定释放出的LDH活性评价细胞膜的完整性[15]。本研究发现,EVO处理HepG2细胞48 h可显著升高上清液中ALT、AST和LDH活性,提示EVO可破坏细胞膜完整性,导致ALT和AST释放增加,对肝细胞具有损伤作用。
分子对接解析了EVO与凋亡相关蛋白caspase-9、caspase-3,自噬相关蛋白p62、Beclin-1、LC3和铁死亡相关蛋白Gpx4的结合情况,结果表明EVO可结合凋亡相关蛋白,但无法与自噬和铁死亡相关蛋白结合,提示EVO所致HepG2细胞毒性作用机制可能涉及细胞凋亡,而与细胞自噬和铁死亡关系不大。
MMP下降作为凋亡的早期指征,是评价线粒体功能的敏感指标。MMP下降可促使线粒体通透性转换孔开放,使细胞色素C从线粒体释放至胞浆,与凋亡蛋白酶活化因子-1结合为多聚体,活化caspase-9前体并进一步激活凋亡执行蛋白caspase-3,导致细胞凋亡[18]。本研究通过JC-10荧光探针染色检测到MMP下降,采用Annexin V-FITC/PI荧光探针染色检测到细胞凋亡率升高,表明EVO可诱导HepG2细胞凋亡。通过Western blot检测凋亡相关蛋白caspase-9和caspase-3的表达水平,发现EVO可下调caspase-3前体蛋白表达,并上调caspase-9和caspase-3剪切蛋白表达。以上结果表明细胞凋亡介导了EVO所致的HepG2细胞毒性。
胆汁淤积性肝损伤是药物性肝损伤中的重要类型。肝细胞至肠道的胆汁转运受阻时,可导致胆汁酸在肝脏中蓄积,引发肝细胞线粒体损伤、氧化应激和细胞死亡[19]。ALP主要经胆汁代谢,当药物引起肝损伤时,胆汁排泄受阻,可引起血清ALP升高,而当肝损伤引起胆红素代谢降低时,可引起血中TBIL的浓度升高,因而ALP和TBIL是反映胆汁淤积型肝损伤的重要标志物。EVO处理HepG2细胞48 h可致ALP活性和TBIL含量升高,提示EVO可能引起胆汁淤积性肝损伤。BSEP和MRP2位于肝细胞胆管侧膜上,是介导游离或结合型胆汁酸外排的重要转运体[20],其中BSEP是关键的胆汁酸转运体,已有多种药物因抑制BSEP功能诱发肝毒性从而导致黑框警告或撤市[21,22]。欧洲药品管理局和国际运输联盟已建议在临床前或临床研究过程中需对药物是否抑制BSEP进行评估[23],可见评估对BSEP的抑制作用对预测药物是否诱导胆汁淤积型肝损伤具有重要意义。本研究发现EVO显著降低HepG2细胞中BSEP和MRP2蛋白表达水平,提示EVO可抑制HepG2细胞BSEP和MRP2转运体表达,使肝细胞内胆汁酸浓度升高,产生胆汁淤积性肝损伤。
综上所述,本研究表明EVO可导致HepG2细胞毒性,其肝细胞毒性机制涉及脂质过氧化损伤、细胞凋亡和胆汁淤积。
参考文献
Scaffold hopping of natural product evodiamine: discovery of a novel antitumor scaffold with excellent potency against colon cancer
[J].
DOI:10.1021/acs.jmedchem.9b01626
PMID:31880942
[本文引用: 1]

Inspired by the natural product evodiamine, a novel antitumor indolopyrazinoquinazolinone scaffold was designed by scaffold hopping. Structure-activity relationship studies led to the discovery of compound, which shows low nanomolar inhibitory activity against the HCT116 cell line. Further antitumor mechanism studies indicated that compound acted by the dual inhibition of topoisomerase 1 and tubulin and induced apoptosis with G2 cell-cycle arrest. The quaternary ammonium salt of compound (compound ) exhibited excellent in vivo antitumor activity (TGI = 66.6%) in the HCT116 xenograft model with low toxicity. Indolopyrazinoquinazolinone derivatives represent promising multitargeting antitumor leads for the development of novel antitumor agents.
Evodiamine inhibits lipopolysaccharide (LPS)-induced inflammation in BV-2 cells via regulating AKT/Nrf2-HO-1/NF-κB signaling axis
[J].DOI:10.1007/s10571-020-00839-w URL [本文引用: 1]
Topoisomerase Ⅰ inhibitor evodiamine acts as an antibacterial agent against drug-resistant Klebsiella pneumoniae
[J].DOI:10.1055/s-00000058 URL [本文引用: 1]
Evodiamine via targeting nNOS and AMPA receptor GluA1 inhibits nitroglycerin-induced migraine-like response
[J].DOI:10.1016/j.jep.2020.112727 URL [本文引用: 1]
Pharmacological basis for the use of evodiamine in Alzheimer’s disease: antioxidation and antiapoptosis
[J].DOI:10.3390/ijms19051527 URL [本文引用: 1]
Molecular mechanisms involved in drug-induced liver injury caused by urate-lowering Chinese herbs: a network pharmacology study and biology experiments
[J].
HepG2 cells simultaneously expressing five P450 enzymes for the screening of hepatotoxicity: identification of bioactivable drugs and the potential mechanism of toxicity involved
[J].
DOI:10.1007/s00204-013-1012-x
PMID:23397584
[本文引用: 1]

Use of the HepG2 cell line to assess hepatotoxicity induced by bioactivable compounds is hampered by their low cytochrome P450 expression. To overcome this limitation, we have used adenoviral transfection to develop upgraded HepG2 cells (ADV-HepG2) expressing the major P450 enzymes involved in drug metabolism (CYP1A2, CYP2D6, CYP2C9, CYP2C19, and CYP3A4) at levels comparable to those of human hepatocytes. The potential utility of this new cell model for the in vitro screening of bioactivable drugs was assessed using a high-content screening assay that we recently developed to simultaneously measure multiple parameters indicative of cell injury. To this end, ADV-HepG2 and HepG2 cells, cultured in 96-well plates, were exposed for 24 h to a wide range of concentrations of 12 bioactivable and 3 non-bioactivable compounds. The cell viability and parameters associated with nuclear morphology, mitochondrial function, intracellular calcium concentration, and oxidative stress indicative of prelethal cytotoxicity and representative of different mechanisms of toxicity were evaluated. Bioactivable compounds showed lower IC(50) values in ADV-HepG2 cells than in HepG2 cells. Moreover, significant differences in the other parameters analyzed were observed between both cell models, while similar effects were observed for non-bioactivable compounds (negative controls). The changes in cell parameters detected in our assay for a given compound are in good agreement with the previously reported toxicity mechanism. Overall, our results indicate that this assay may be a suitable new in vitro approach for early screening of compounds to identify bioactivable hepatotoxins and the mechanism(s) involved in their toxicity.
Surflex: fully automatic flexible molecular docking using a molecular similarity-based search engine
[J].DOI:10.1021/jm020406h URL [本文引用: 1]
Superoxide dismutase as a target for the selective killing of cancer cells
[J].DOI:10.1038/35030140 URL [本文引用: 1]
Biological markers of oxidative stress: applications to cardiovascular research and practice
[J].DOI:10.1016/j.redox.2013.07.006 URL [本文引用: 1]
Bile-acid-induced cell injury and protection
[J].DOI:10.3748/wjg.15.1677 URL [本文引用: 1]
Enterohepatic transport of bile salts and genetics of cholestasis
[J].
Systems pharmacology modeling predicts delayed presentation and species differences in bile acid-mediated Troglitazone hepatotoxicity
[J].
DOI:10.1038/clpt.2014.158
PMID:25068506
[本文引用: 1]

Troglitazone (TGZ) causes delayed, life-threatening drug-induced liver injury in some patients but was not hepatotoxic in rats. This study investigated altered bile acid homeostasis as a mechanism of TGZ hepatotoxicity using a systems pharmacology model incorporating drug/metabolite disposition, bile acid physiology/pathophysiology, hepatocyte life cycle, and liver injury biomarkers. In the simulated human population, TGZ (200-600 mg/day × 6 months) resulted in delayed increases in serum alanine transaminase >3× the upper limit of normal in 0.3-5.1%, with concomitant bilirubin elevations >2× the upper limit of normal in 0.3-3.6%, of the population. By contrast, pioglitazone (15-45 mg/day × 6 months) did not elicit hepatotoxicity, consistent with clinical data. TGZ was not hepatotoxic in the simulated rat population. In summary, mechanistic modeling based only on bile acid effects accurately predicted the incidence, delayed presentation, and species differences in TGZ hepatotoxicity, in addition to predicting the relative liver safety of pioglitazone. Systems pharmacology models integrating physiology and experimental data can evaluate drug-induced liver injury mechanisms and may be useful to predict the hepatotoxic potential of drug candidates.
The endothelin antagonist bosentan inhibits the cana-licular bile salt export pump: a potential mechanism for hepatic adverse reactions
[J].During clinical trials bosentan, the first orally active endothelin receptor antagonist, caused asymptomatic transaminase elevations in some patients. In this study we investigated whether inhibition of the hepatocanalicular bile salt export pump (rodents, Bsep; humans, BSEP ABCB11) could account for bosentan-induced liver injury.We reanalyzed the safety database of the bosentan trials for cholestatic liver injury, determined the cholestatic potency of bosentan in the rat, and studied the effects of bosentan and its metabolites on Bsep-mediated taurocholate transport in vitro.Bosentan caused dose-dependent and reversible liver injury in 2% to 18% of patients and caused a significant increase of serum bile salt levels (P <.01). Concomitant administration of glyburide (INN, glibenclamide) enhanced the cholestatic potency of bosentan. Similar effects were seen in rats, in which serum bile salt levels were increased by glyburide less than by bosentan, which increased the levels less than a combination of bosentan and glyburide. In vitro, Bsep-mediated taurocholate transport was inhibited by bosentan (inhibition constant, approximately 12 micromol/L) and metabolites (inhibition constant, approximately 8.5 micromol/L for metabolite Ro 47-8634).These results indicate that bosentan-induced liver injury is mediated, at least in part, by inhibition of Bsep/BSEP-causing intracellular accumulation of cytotoxic bile salts and bile salt induced liver cell damage. The data further emphasize the pathophysiologic importance of drug-Bsep interactions in acquired forms of cholestatic liver injury.
Current concepts in drug-induced bile salt export pump (BSEP) interference
[J].