北京大学学报(医学版) ›› 2021, Vol. 53 ›› Issue (6): 1107-1114. doi: 10.19723/j.issn.1671-167X.2021.06.017
吴茱萸碱对HepG2细胞毒性及其机制
高亚东1,朱安1,李璐迪1,张涛1,王硕1,单丹萍1,李盈姿1,王旗1,2,3,△( )
)
- 1.北京大学公共卫生学院毒理学系,北京 100191
2.国家中医药管理局中药配伍减毒重点研究室,北京 100191
3.食品安全毒理学研究与评价北京市重点实验室,北京 100191
Cytotoxicity and underlying mechanism of evodiamine in HepG2 cells
GAO Ya-dong1,ZHU An1,LI Lu-di1,ZHANG Tao1,WANG Shuo1,SHAN Dan-ping1,LI Ying-zi1,WANG Qi1,2,3,△( )
)
- 1. Department of Toxicology, Peking University School of Public Health, Beijing 100191, China
2. Key Laboratory of State Administration of Traditional Chinese Medicine for Compatibility Toxicology, Beijing 100191, China
3. Beijing Key Laboratory of Toxicological Research and Risk Assessment for Food Safety, Beijing 100191, China
摘要:
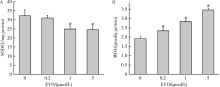
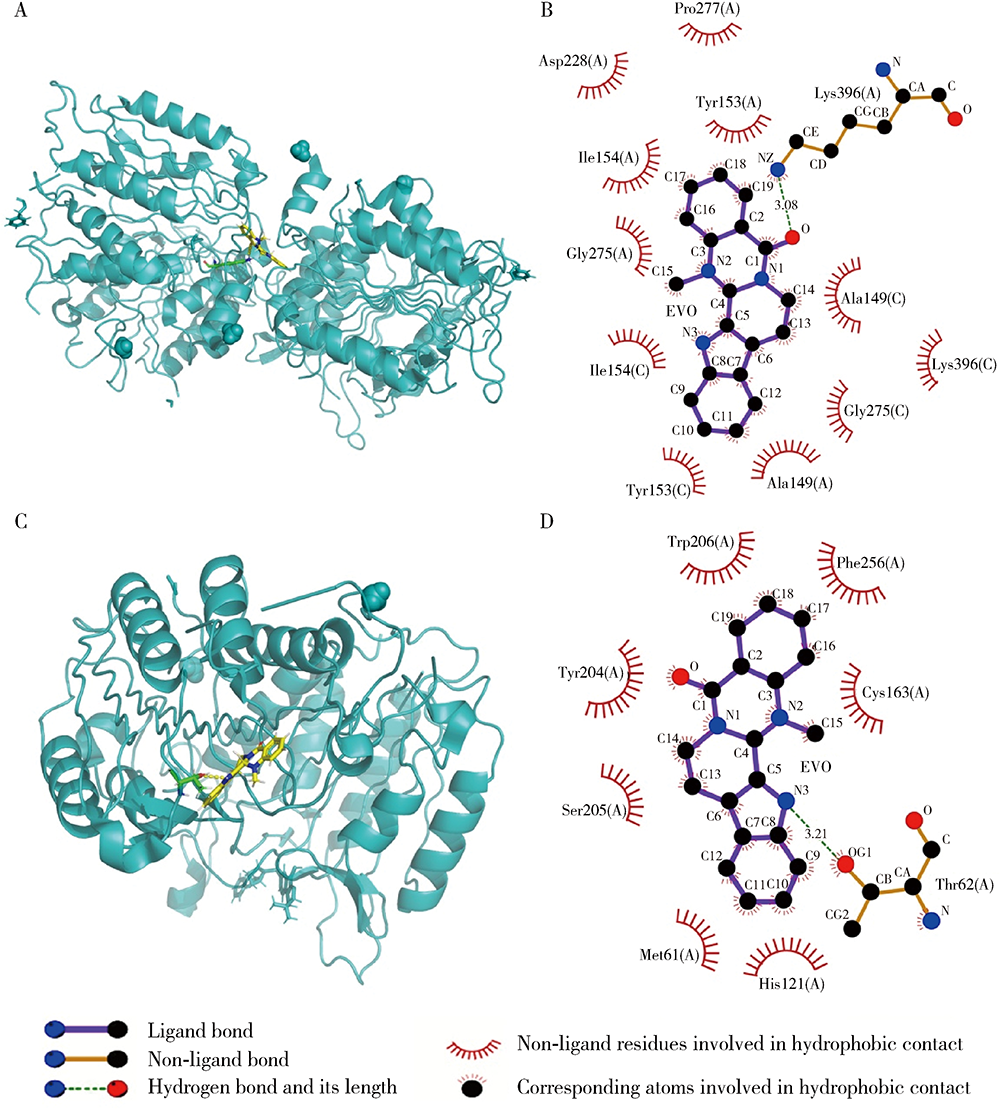
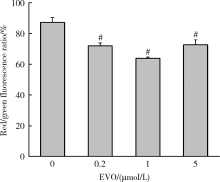
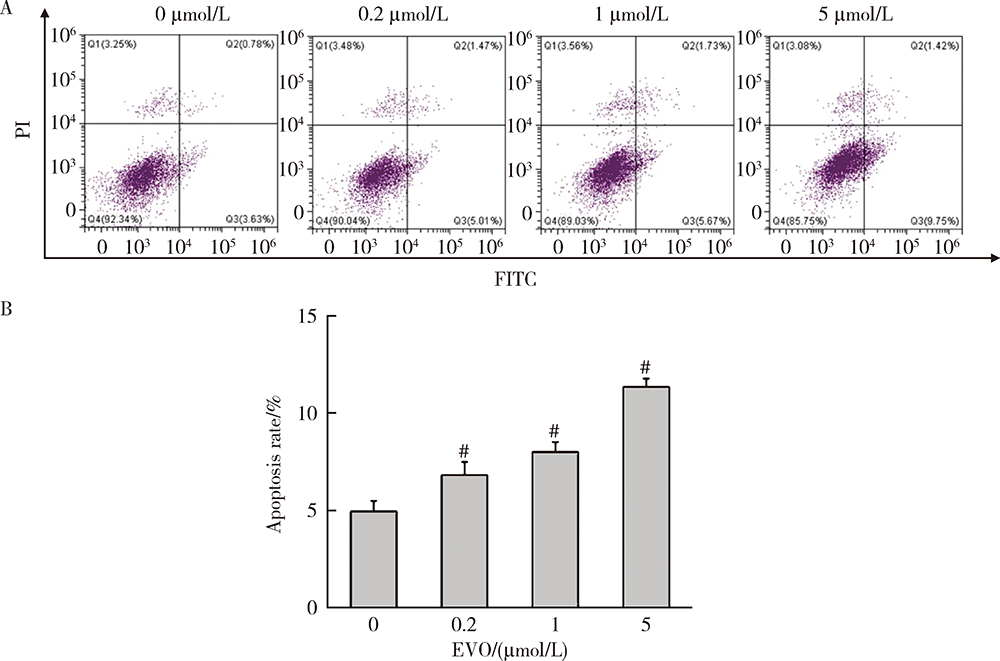
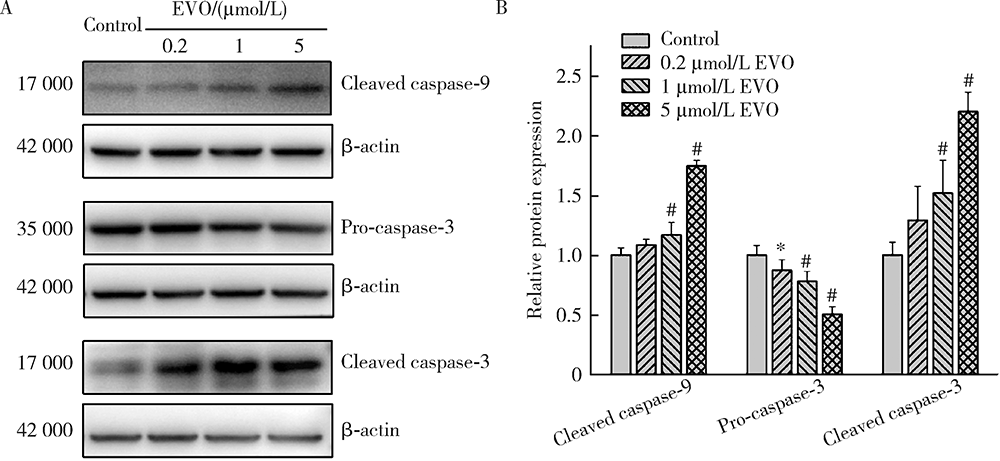
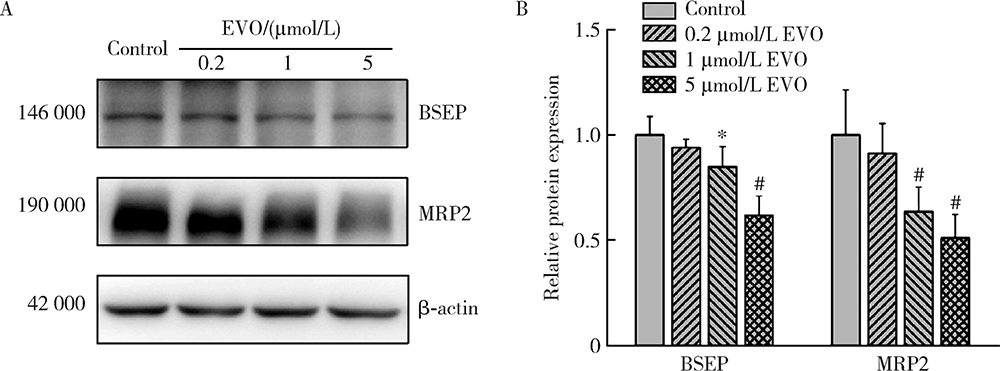
目的:研究吴茱萸碱(evodiamine,EVO)的肝细胞毒性及其机制。方法:将0.04~25 μmol/L EVO分别作用于HepG2细胞24、48和72 h,用细胞增殖及毒性检测(cell counting kit-8,CCK-8)法检测细胞存活率。0.2、1和5 μmol/L EVO处理HepG2细胞48 h,多功能酶标仪分别检测细胞培养上清液中谷丙转氨酶(alanine transaminase,ALT)、谷草转氨酶(aspartate aminotransferase,AST)、乳酸脱氢酶(lactate dehydrogenase,LDH)、碱性磷酸酶(alkaline phosphatase,ALP)活性,以及总胆红素(total bilirubin,TBIL)含量。用多功能酶标仪检测HepG2细胞内超氧化物歧化酶(superoxide dismutase,SOD)活性和丙二醛(malondialdehyde,MDA)含量。用分子对接探究EVO与凋亡、自噬和铁死亡相关蛋白结合情况。用线粒体膜电位荧光探针(superior alternative to JC-1,JC-10)和异硫氰酸荧光素标记的膜联蛋白/碘化丙啶(annexin V-fluorescein isothiocyanate/propidium iodide,Annexin V-FITC/PI)对HepG2细胞进行染色,流式细胞仪分别检测细胞线粒体膜电位(mitochondrial membrane potential,MMP)和细胞凋亡。用Western blot检测HepG2细胞凋亡相关蛋白caspase-9,caspase-3以及胆汁酸转运体胆盐输出泵(bile salt export pump,BSEP)和多耐药相关蛋白2(multidrug resistance-associated protein 2,MRP2)的表达水平。结果:0.04~25 μmol/L EVO可降低HepG2细胞存活率,具有时间和剂量依赖关系。EVO处理HepG2细胞24、48和72 h的半数抑制浓度(half maximal inhibitory concentration,IC50)分别为85.3、6.6和4.7 μmol/L。0.2、1和5 μmol/L EVO作用HepG2细胞48 h,细胞培养上清液中ALT、AST、LDH、ALP活性升高,TBIL含量增加。EVO可降低细胞中SOD活性,增加MDA含量。分子对接结果显示EVO与凋亡相关蛋白结合情况较好,JC-10和Annexin V-FITC/PI染色发现EVO可降低MMP,增加细胞凋亡率。Western blot结果表明EVO可上调caspase-9剪切蛋白和caspase-3剪切蛋白表达,并下调caspase-3前体蛋白、BSEP和MRP2蛋白表达。结论:0.2、1和5 μmol/L的EVO具有肝细胞毒性,其毒性机制可能涉及脂质过氧化损伤、细胞凋亡和胆汁淤积。
中图分类号:
- R114
| [1] | 国家药典委员会. 中华人民共和国药典一部[M]. 11版. 北京: 中国医药科技出版社, 2020: 178. |
| [2] | 刘颖, 杨润芳, 夏祺悦, 等. 吴茱萸醇提物重复给药的靶器官毒性研究[J]. 现代预防医学, 2015, 42(14):2600-2603. |
| [3] | 黄伟, 李晓骄阳, 孙蓉. 吴茱萸水提组分多次给药对小鼠肝毒性的“量-时-毒”关系研究[J]. 中国中药杂志, 2012, 37(15):2223-2227. |
| [4] |
Wang L, Fang K, Cheng J, et al. Scaffold hopping of natural product evodiamine: discovery of a novel antitumor scaffold with excellent potency against colon cancer[J]. J Med Chem, 2020, 63(2):696-713.
doi: 10.1021/acs.jmedchem.9b01626 pmid: 31880942 |
| [5] |
Meng T, Fu S, He D, et al. Evodiamine inhibits lipopolysaccharide (LPS)-induced inflammation in BV-2 cells via regulating AKT/Nrf2-HO-1/NF-κB signaling axis[J]. Cell Mol Neurobiol, 2020, 41(1):115-127.
doi: 10.1007/s10571-020-00839-w |
| [6] |
Wu JY, Chang MC, Chen CS, et al. Topoisomerase Ⅰ inhibitor evodiamine acts as an antibacterial agent against drug-resistant Klebsiella pneumoniae[J]. Planta Med, 2013, 79(1):27-29.
doi: 10.1055/s-00000058 |
| [7] |
Lin J, Zhang X, Li C, et al. Evodiamine via targeting nNOS and AMPA receptor GluA1 inhibits nitroglycerin-induced migraine-like response[J]. J Ethnopharmacol, 2020, 254:112727.
doi: 10.1016/j.jep.2020.112727 |
| [8] |
Zhang Y, Wang J, Wang C, et al. Pharmacological basis for the use of evodiamine in Alzheimer’s disease: antioxidation and antiapoptosis[J]. Int J Mol Sci, 2018, 19(5):1527.
doi: 10.3390/ijms19051527 |
| [9] | Li F, Dong YZ, Zhang D, et al. Molecular mechanisms involved in drug-induced liver injury caused by urate-lowering Chinese herbs: a network pharmacology study and biology experiments[J]. PLoS One, 2019, 14(5):e216948. |
| [10] | 黄伟, 孙蓉. 吴茱萸水提组分多次给药致小鼠肝毒性氧化损伤机制研究[J]. 中药药理与临床, 2012, 28(5):114-116. |
| [11] | 蔡卿嫣. 吴茱萸水提物的大鼠肝毒性及其线粒体损伤机制研究[D]. 广州: 广州中医药大学, 2014. |
| [12] |
Tolosa L, Gómez-Lechón MJ, Pérez-Cataldo G, et al. HepG2 cells simultaneously expressing five P450 enzymes for the screening of hepatotoxicity: identification of bioactivable drugs and the potential mechanism of toxicity involved[J]. Arch Toxicol, 2013, 87(6):1115-1127.
doi: 10.1007/s00204-013-1012-x pmid: 23397584 |
| [13] |
Jain AN. Surflex: fully automatic flexible molecular docking using a molecular similarity-based search engine[J]. J Med Chem, 2003, 46(4):499-511.
doi: 10.1021/jm020406h |
| [14] | 李文兰, 孙向明, 陈晨, 等. 基于UPLC-Q-TOF MS的吴茱萸致肝毒性部位及入血成分分析[J]. 质谱学报, 2017, 38(3):282-293. |
| [15] | 高绪聪, 柴振海, 张宗鹏. 药物性肝损伤的生物标志物及其评价的研究进展[J]. 中国药理学与毒理学杂志, 2012, 5(26):692-696. |
| [16] |
Huang P, Feng L, Oldham EA, et al. Superoxide dismutase as a target for the selective killing of cancer cells[J]. Nature, 2000, 407(6802):390-395.
doi: 10.1038/35030140 |
| [17] |
Ho E, Karimi Galougahi K, Liu C, et al. Biological markers of oxidative stress: applications to cardiovascular research and practice[J]. Redox Biol, 2013, 1(1):483-491.
doi: 10.1016/j.redox.2013.07.006 |
| [18] | 刘晓婷, 王延让, 张明. 线粒体介导细胞凋亡的研究进展[J]. 环境与健康杂志, 2013, 30(2):182-185. |
| [19] |
Perez MJ, Briz O. Bile-acid-induced cell injury and protection[J]. World J Gastroenterol, 2009, 15(14):1677-1689.
doi: 10.3748/wjg.15.1677 |
| [20] |
Pauli-Magnus C, Stieger B, Meier Y, et al. Enterohepatic transport of bile salts and genetics of cholestasis[J]. J Hepatol, 2005, 43(2):342-357.
pmid: 15975683 |
| [21] |
Yang K, Woodhead JL, Watkins PB, et al. Systems pharmacology modeling predicts delayed presentation and species differences in bile acid-mediated Troglitazone hepatotoxicity[J]. Clin Pharmacol Ther, 2014, 96(5):589-598.
doi: 10.1038/clpt.2014.158 pmid: 25068506 |
| [22] |
Fattinger K. The endothelin antagonist bosentan inhibits the cana-licular bile salt export pump: a potential mechanism for hepatic adverse reactions[J]. Clin Pharmacol Ther, 2001, 69(4):223-231.
pmid: 11309550 |
| [23] | Kenna JG. Current concepts in drug-induced bile salt export pump (BSEP) interference[J]. Curr Protoc Toxicol, 2014, 61(1):1-15. |
| [1] | 刘耘充,吴宗龙,葛力源,杜坦,吴雅倩,宋一萌,刘承,马潞林. 肾透明细胞癌中核蛋白1对阿昔替尼耐药的作用及机制[J]. 北京大学学报(医学版), 2023, 55(5): 781-792. |
| [2] | 娄雪,廖莉,李兴珺,王楠,刘爽,崔若玫,徐健. 类风湿关节炎患者外周血TWEAK基因启动子区甲基化状态及其表达[J]. 北京大学学报(医学版), 2021, 53(6): 1020-1025. |
| [3] | 苏雷震,陈洁,李显,季平. 沙利霉素对口腔鳞癌细胞增殖和凋亡的影响[J]. 北京大学学报(医学版), 2020, 52(5): 902-906. |
| [4] | 耿良,吕静,范敬. 肺瘤平膏联合环磷酰胺化疗对肺癌的抑瘤作用和酸性微环境的影响[J]. 北京大学学报(医学版), 2020, 52(2): 247-253. |
| [5] | 孙静,宋卫东,闫思源,席志军. 氯喹抑制肾癌细胞活性促进舒尼替尼诱导的细胞凋亡[J]. 北京大学学报(医学版), 2018, 50(5): 778-784. |
| [6] | 李满,李圆,孙麟,宋君来,吕聪. 高迁移率族蛋白B1通过调节Bcl-2和Bax蛋白表达促进氧糖剥夺/复氧星形胶质细胞的凋亡[J]. 北京大学学报(医学版), 2018, 50(5): 785-791. |
| [7] | 王昊,陈亮,叶小云. 雷公藤甲素对TM4细胞氧化应激及PI3K/AKT通路的影响[J]. 北京大学学报(医学版), 2018, 50(4): 607-612. |
| [8] | 王玉洁,郭向阳,王军. 重复异丙酚麻醉对新生大鼠海马细胞凋亡及远期学习记忆能力的影响[J]. 北京大学学报(医学版), 2017, 49(2): 310-314. |
| [9] | 杨光,程庆砾,李春霖,贾雅丽,岳文,裴雪涛,刘洋,赵佳慧,杜婧,敖强国. 高糖减弱肾组织干细胞条件培养液对缺氧损伤肾小管上皮细胞的修复作用[J]. 北京大学学报(医学版), 2017, 49(1): 125-130. |
| [10] | 曹珮,姜学军,席志军. 舒尼替尼通过抑制Akt/mTOR信号通路诱导肾癌细胞自噬[J]. 北京大学学报(医学版), 2016, 48(4): 584-589. |
| [11] | 李刚,张洪宪,王云鹏,张径,洪锴,田晓军,马潞林. 间苯三酚对大鼠肾缺血再灌注损伤的保护作用[J]. 北京大学学报(医学版), 2015, 47(5): 743-748. |
| [12] | 郑少强, 陈雪, 王雅杰, 安立新. 七氟烷对幼鼠脑细胞凋亡和远期学习记忆功能的影响[J]. 北京大学学报(医学版), 2015, 47(4): 674-678. |
| [13] | 温静,程庆砾,马强,齐云,赵佳慧,杜婧,王小丹,刘胜,李美花,张晓英. 肾组织干细胞对人肾小管上皮细胞损伤修复的作用[J]. 北京大学学报(医学版), 2013, 45(4): 619-. |
| [14] | 杨轩, 袁栋栋, 姜学军, 席志军. 顺铂通过诱导膀胱癌细胞自噬促进细胞凋亡[J]. 北京大学学报(医学版), 2013, 45(2): 221-. |
| [15] | 刘慧琳, 田勍, 洪天配, 刘桂花, 潘欢, 王海宁, 高洪伟. 脓毒症患者中血清程序化细胞死亡因子5水平的变化[J]. 北京大学学报(医学版), 2013, 45(2): 238-. |
|
||