北京大学学报(医学版) ›› 2019, Vol. 51 ›› Issue (6): 1078-1084. doi: 10.19723/j.issn.1671-167X.2019.06.018
化疗后结直肠癌转移瘤钙化CT影像学表现与化疗反应之间的关系
- 1. 四川大学华西医院放射科,成都 610041
2. 重庆大学附属肿瘤医院影像科,重庆 400030
3. 四川大学华西医院肿瘤中心,成都 610041
Relationship between the CT features of colorectal cancer metastases calcification and tumor response to chemotherapy
Jing ZHANG1,2,Yu-wen ZHOU3,Meng QIU3,Lan-qing YANG1,Bing WU1,△( )
)
- 1. Departments of Radiology, West China Hospital,Sichuan University, Chengdu 610041, China
2. Departments of Radiology, Chongqing University Cancer Hospital, Chongqing 400030, China
3. Departments of Oncology, West China Hospital,Sichuan University, Chengdu 610041, China
摘要:
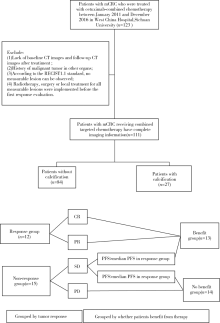
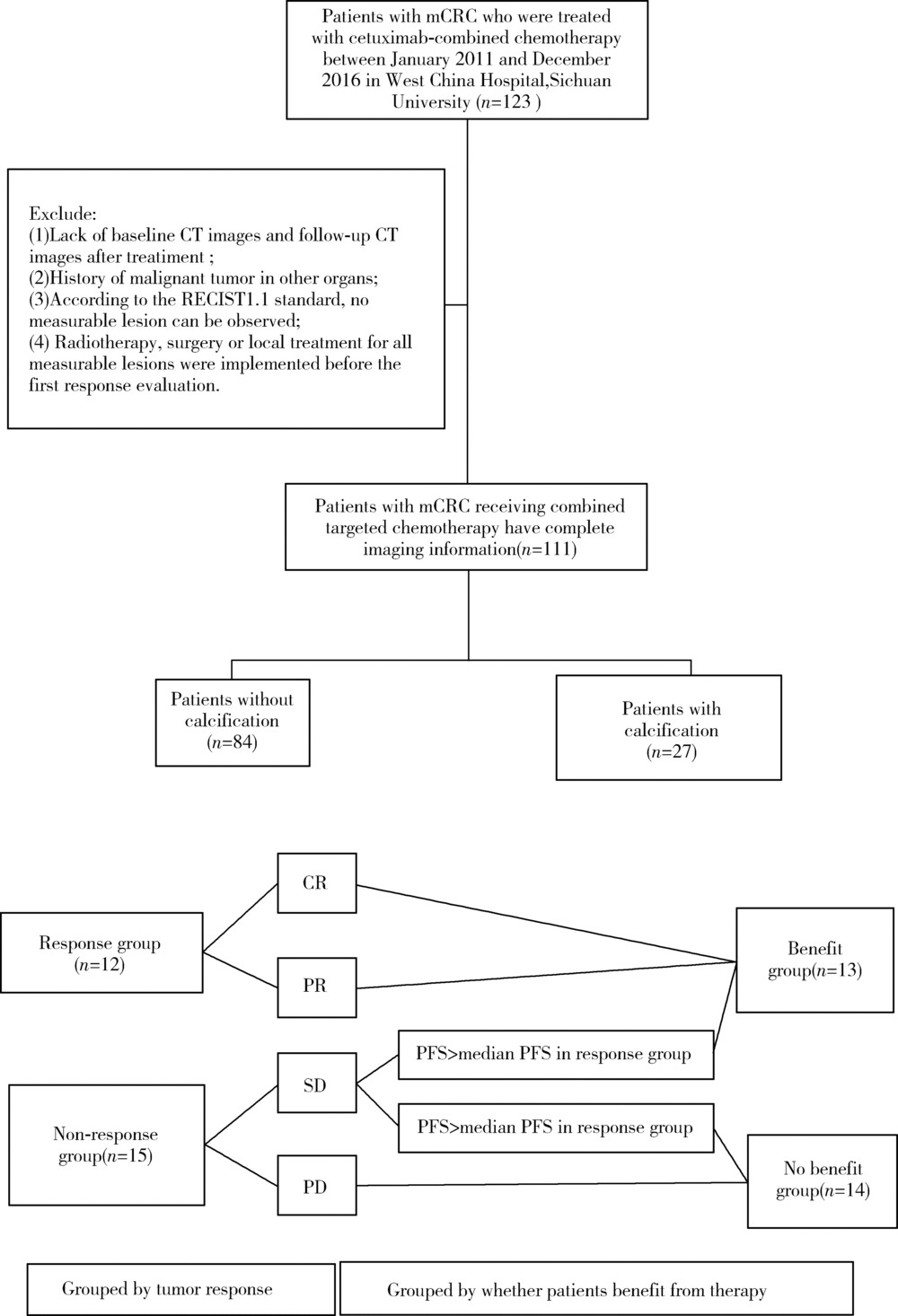
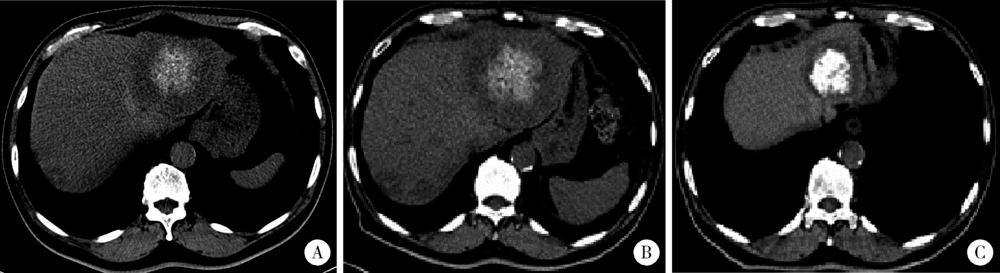
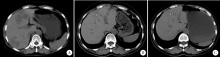
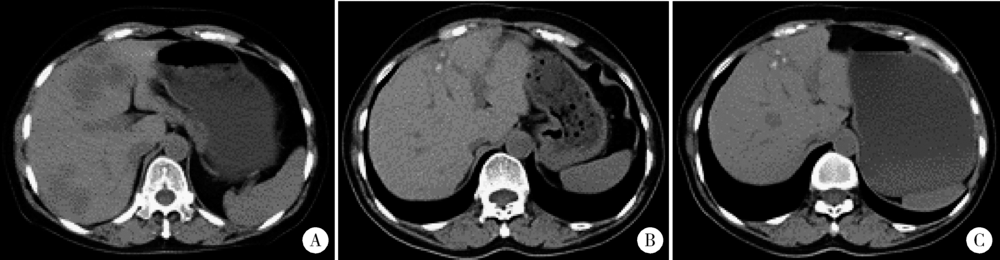
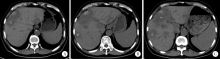
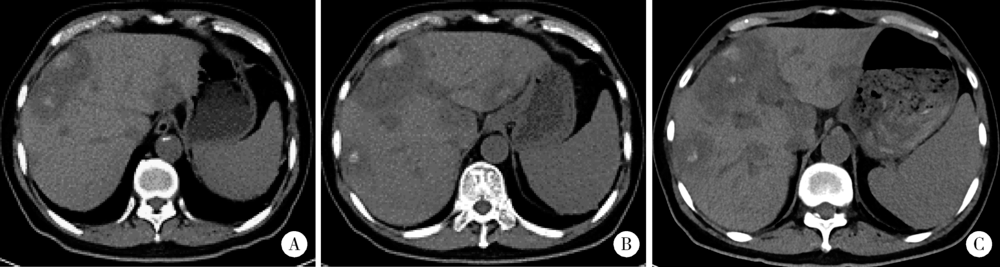
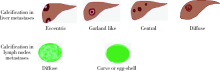
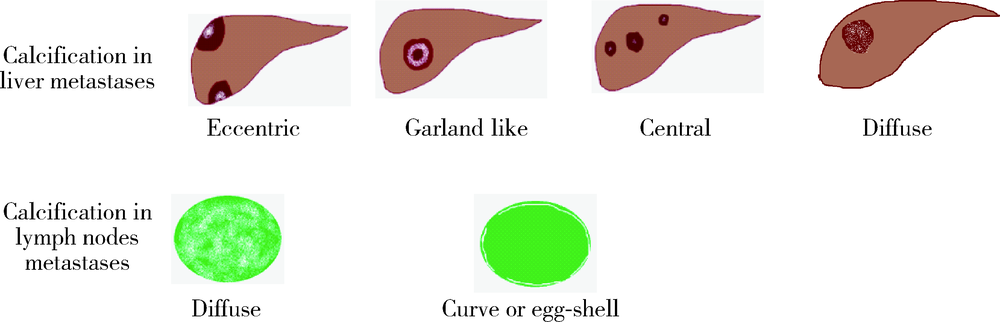
目的 探究结直肠癌转移灶在联合西妥昔单抗靶向化疗后钙化的CT特征与其治疗疗效的关系。方法 回顾性分析2011年1月至2016年12月接受过联合西妥昔单抗靶向化疗且有完整资料的转移性结直肠癌钙化患者。两位影像科医生对患者治疗前后的肿瘤钙化的发生、钙化特征,以及治疗疗效评价进行独立评估。参照《实体肿瘤疗效评价标准(1.1版本)》 对患者的最佳疗效评价进行记录:(1)将完全缓解(complete response,CR)和部分缓解(partial response,PR)归为治疗有反应组,将疾病稳定(stable disease,SD)和疾病进展(progressive disease,PD)归为治疗无反应组;(2)对于疗效评价为SD的患者,由于无进展生存时间(progress free survival,PFS)较长的患者可以认为能从治疗中获益,因此根据PFS长短对其进行进一步分组,将PFS大于治疗有反应组中位PFS的患者与疗效评价为CR或PR的患者归为治疗获益组,余者归为治疗未获益组。对比分析患者转移瘤钙化的不同影像学特征(钙化形态、最大钙化密度、钙化密度-时间增长斜率)的差异。结果 在所有符合要求的111名患者中,出现肿瘤钙化的患者总计27例,共涉及30个部位,其中肝脏转移灶钙化患者19个(63.3%),淋巴结转移8个(26.7%),肺转移2个(6.7%),皮下转移1个(3.3%)。治疗有反应组12例,治疗无反应组15例;治疗获益组13例,治疗无获益组14例。治疗有反应组对比无反应组有较高的钙化密度-时间增长斜率,治疗获益组表现为钙化灶数量增加的比例(61.5%)较治疗无获益组(14.3%)高(P=0.018),最大钙化密度在各分组间差异均无统计学意义。肝转移瘤钙化灶均为无定形钙化,呈中心性钙化(占36.8%)、偏心性钙化(占36.8%)以及花环状钙化(占15.8%)和弥漫性钙化(占10.6%)。淋巴结转移灶可呈弥漫型(占75.0%), 以及曲线或壳状钙化(占25.0%),在各分组间差异无统计学意义。结论 在接受联合西妥昔单抗靶向化疗的晚期结直肠癌发生钙化的患者中,密度增长快、钙化数量的增加可能成为治疗疗效有效的影像学特征,最大钙化密度和钙化形态与疗效无明显关系。
中图分类号:
- R445
| [1] | Chen W, Zheng R, Baade PD , et al. Cancer statistics in China, 2015[J]. CA Cancer J Clin, 2016,66(2):115-132. |
| [2] | Siegel RL, Miller KD, Jemal A . Cancer statistics, 2018[J]. CA Cancer J Clin, 2018,68(1):7-30. |
| [3] | Eisenhauer EA, Therasse P, Bogaerts J , et al. New response eva-luation criteria in solid tumours: Revised RECIST guideline (version 1.1)[J]. Eur J Cancer, 2009,45(2):228-247. |
| [4] | Easson AM, Barron PT, Cripps C , et al. Calcification in colorectal hepatic metastases correlates with longer survival[J]. J Surg Oncol, 1996,63(4):221-225. |
| [5] | Hale HL, Husband JE, Gossios K , et al. CT of calcified liver metastases in colorectal carcinoma[J]. Clin Radiol, 1998,53(10):735-741. |
| [6] | 姜昊, 姜慧杰, 潘文彬 , 等. 不同来源肝转移瘤多层螺旋CT影像学特征的分析[J]. 中华结直肠疾病电子杂志, 2017,6(1):41-45. |
| [7] | Roy B, Verma S, Awasthi R , et al. Correlation of phase values with CT Hounsfield and R2*values in calcified neurocysticercosis[J]. J Magn Reson Imaging, 2011,34(5):1060-1064. |
| [8] | Giachelli CM . Ectopic calcification:Gathering hard facts about soft tissue mineralization[J]. Am J Pathol, 1999,154(3):671-675. |
| [9] | Agarwal A, Yeh BM, Breiman RS , et al. Peritoneal calcification: Causes and distinguishing features on CT[J]. AJR Am J Roentgenol, 2004,182(2):441-445. |
| [10] | 邓祥春, 郑波, 童朝阳 , 等. 多层螺旋CT对黏液性与非黏液性结直肠癌的鉴别诊断价值[J]. 中国CT和MRI杂志, 2015,13(8):80-83. |
| [11] | Sweeney DJ, Low VH, Robbins PD , et al. Calcified lymph node metastases in adenocarcinoma of the colon[J]. Australas Radiol, 1994,38(3):233-234. |
| [12] | Caskey CI, Fishman EK . Computed tomography of calcified meta-stases to skeletal muscle from adenocarcinoma of the colon[J]. J Comput Tomogr, 1988,12(3):199-202. |
| [13] | Yoshikawa H, Kameyama M, Ueda T , et al. Ossifying intramuscular metastasis from colon cancer: Report of a case[J]. Dis Colon Rectum, 1999,42(9):1225-1227. |
| [14] | Yu MH, Kim YJ, Park HS , et al. Imaging patterns of intratumoral calcification in the abdominopelvic cavity[J]. Korean J Radiol, 2017,18(2):323-335. |
| [15] | Günhan-Bilgen I, Oktay A . Management of microcalcifications developing at the lumpectomy bed after conservative surgery and radiation therapy[J]. AJR Am J Roentgenol, 2007,188(2):393-398. |
| [16] | Goyer P, BenoistE S, Julie C , et al. Complete calcification of colorectal liver metastases on imaging after chemotherapy does not indicate sterilization of disease[J]. J Visc Surg, 2012,149(4):E271-E274. |
| [17] | Cheng JM, Tirumani SH, Kim KW , et al. Malignant abdominal rocks: where do they come from?[J]. Cancer Imaging, 2013,13(4):527-539. |
| [1] | 穆东亮,薛铖,安彬,王东信. 硬膜外阻滞与结直肠癌患者术后远期生存状态的关系:一项倾向性评分匹配的回顾性研究[J]. 北京大学学报(医学版), 2021, 53(6): 1152-1158. |
| [2] | 高乐,于树青,杨继春,马峻岭,詹思延,孙凤. 全球结直肠癌筛查指南的质量评价[J]. 北京大学学报(医学版), 2019, 51(3): 548-555. |
| [3] | 肖洋,杜瑶瑶,高成,孔炜. microRNA在高磷诱导血管平滑肌细胞钙化早期的动态变化[J]. 北京大学学报(医学版), 2016, 48(5): 756-765. |
| [4] | 李斌斌, 李铁军. 颌骨牙本质生成性影细胞瘤的复发与恶变[J]. 北京大学学报(医学版), 2011, 43(1): 48-51. |
| [5] | 张宝红, 唐朝枢, 杜军保. 钙化血管细胞凋亡基因的变化[J]. 北京大学学报(医学版), 2003, 35(1): 45-49. |
|