北京大学学报(医学版) ›› 2021, Vol. 53 ›› Issue (6): 1171-1177. doi: 10.19723/j.issn.1671-167X.2021.06.027
甲磺酸去铁胺促进大鼠颅骨临界骨缺损血管化骨再生的早期连续观察
- 北京大学口腔医学院·口腔医院,修复科 国家口腔医学中心 国家口腔疾病临床医学研究中心 口腔数字化医疗技术和材料国家工程实验室,北京 100081
Early constant observation of the effect of deferoxamine mesylate on improvement of vascularized bone regeneration in SD rat skull critical size defect model
DU Wen-yu,YANG Jing-wen( ),JIANG Ting(
),JIANG Ting( )
)
- Department of Prosthodontics, Peking University School and Hospital of Stomatology & National Center of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology, Beijing 100081, China
摘要:
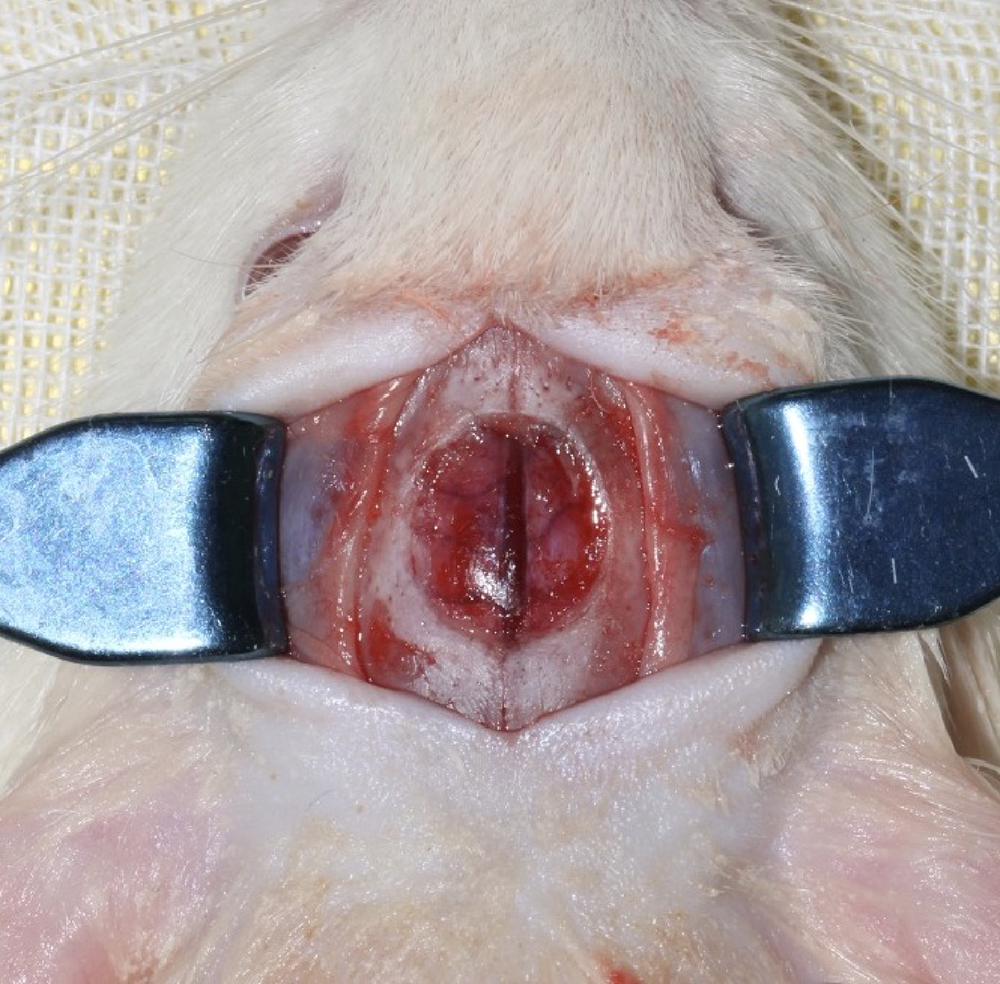
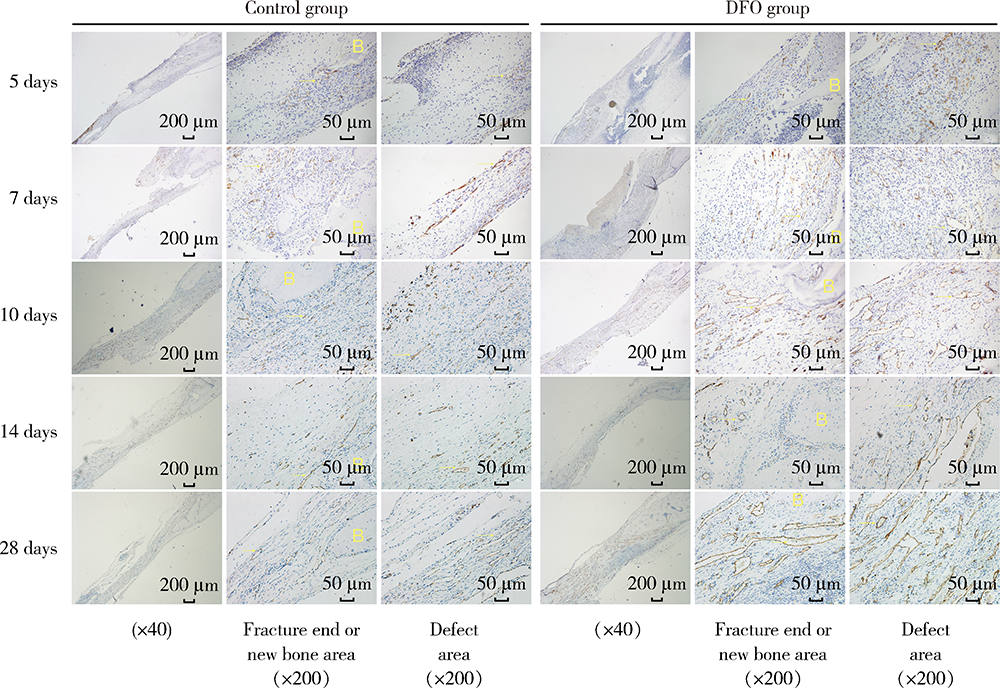
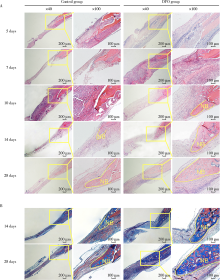
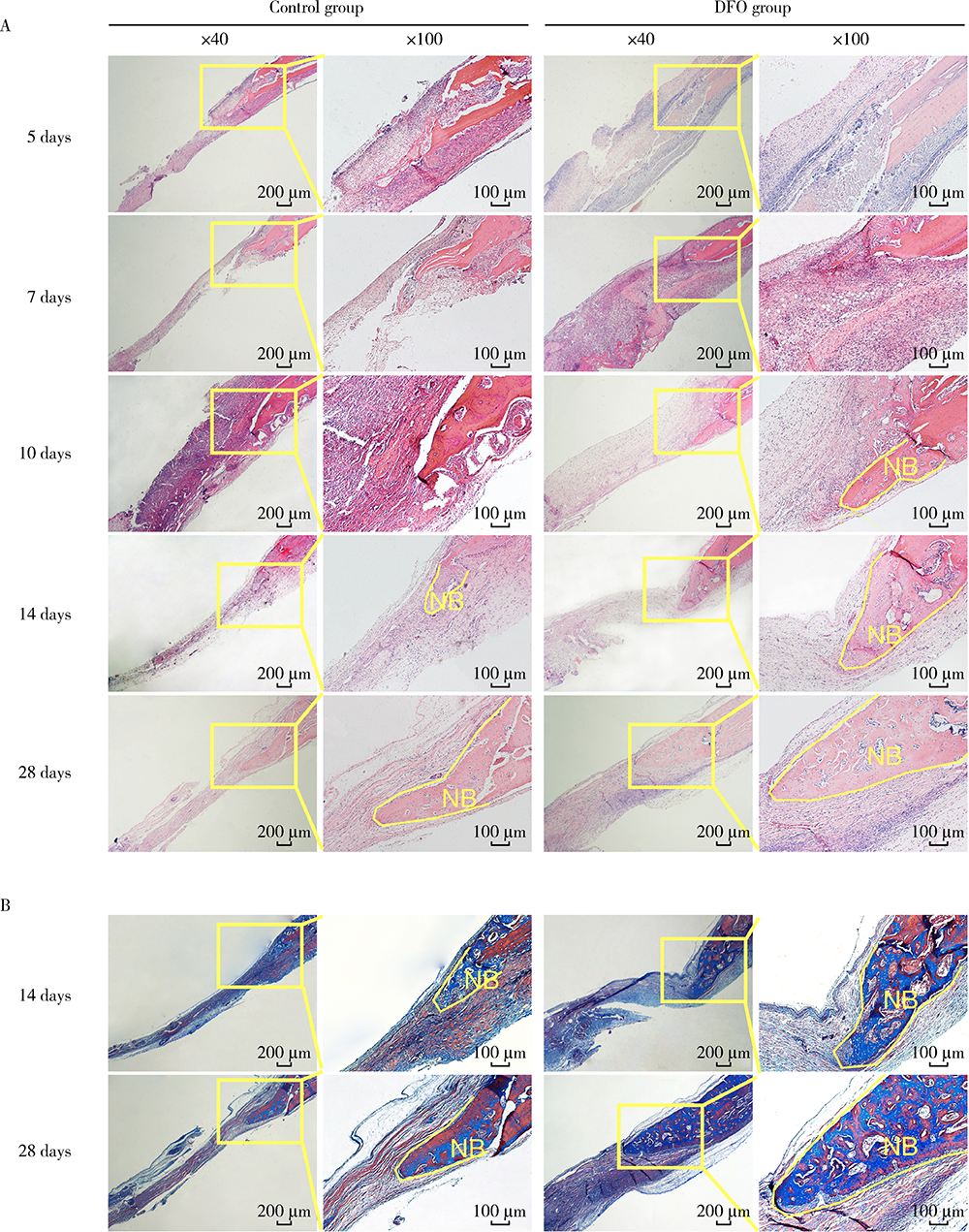
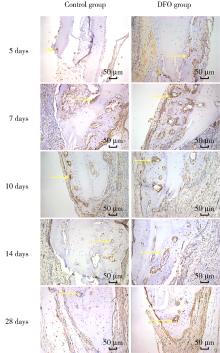
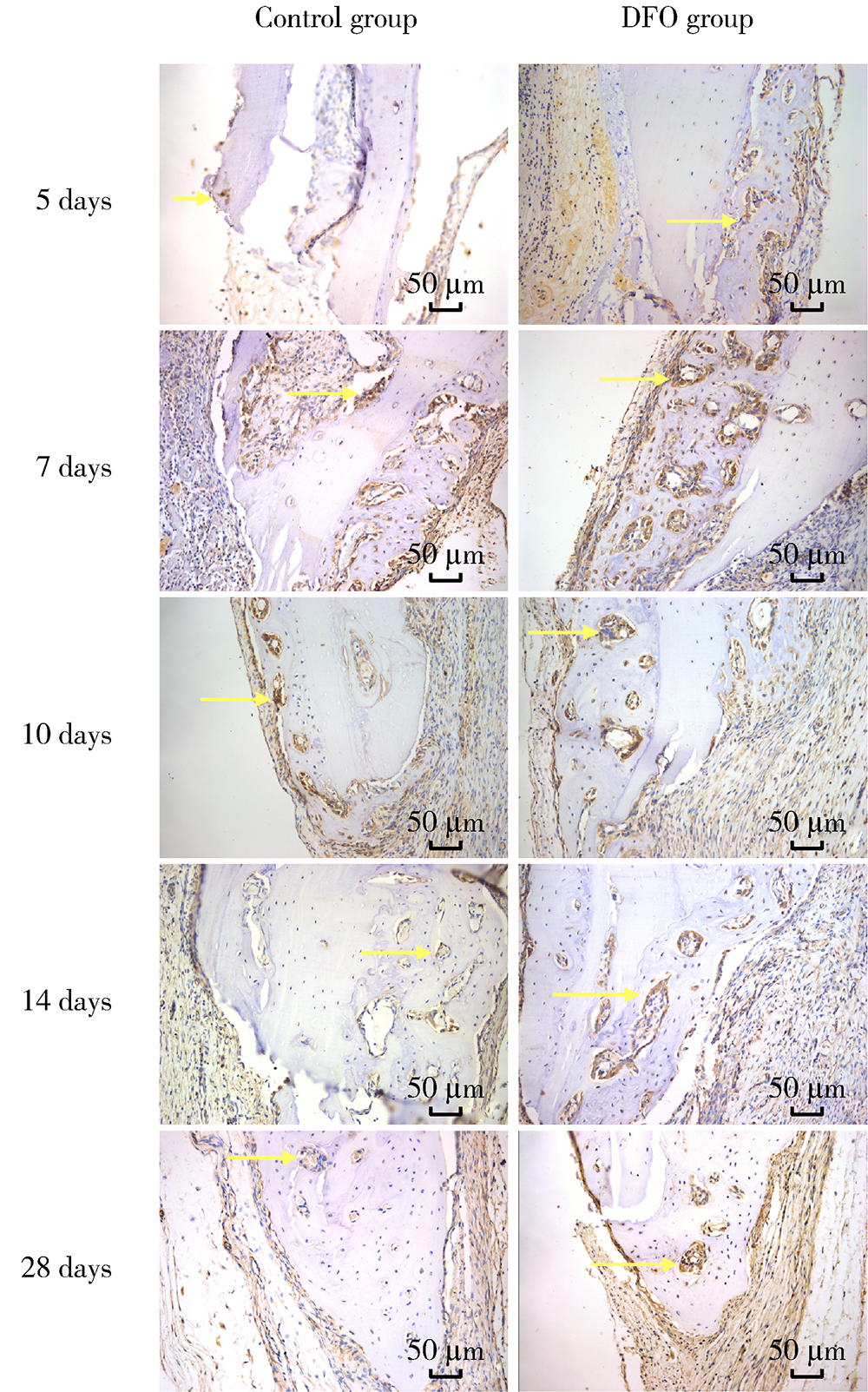
目的:连续观察骨缺损愈合早期血管样组织和骨样组织的变化过程,初步了解局部施用甲磺酸去铁胺(deferoxamine mesylate,DFO)对血管化及骨再生的作用,验证DFO维持缺氧诱导因子-1α(hypoxia inducible factor-1α, HIF-1α)活性的能力。方法:构建30只6~8周龄雄性SD大鼠颅骨缺损模型,随机分为DFO实验组和生理盐水对照组,于颅骨缺损后第4天分别局部注射200 μmol/L DFO溶液300 μL或生理盐水300 μL,并于缺损后第5、7、10、14和28天,每组每次处死3只大鼠。采用CD31免疫组织化学染色检测血管数量,采用HE染色、Masson染色观察成骨及矿化情况,以HIF-1α免疫组化染色检测HIF-1α蛋白相对表达量。结果:术后第5、7、10、14和28天,实验组血管数量(个)分别为:30.40±12.15、62.00±17.87、73.43±15.63、40.00±7.84和48.71±11.64,对照组血管数量(个)分别为:18.75±6.63、19.13±2.80、51.35±16.21、27.18±7.32和30.88±13.43,各时间点实验组血管数量均显著多于对照组(P<0.05);术后第14和28天,实验组新生骨组织较多,新生骨组织矿化百分比分别为(27.73±5.93)%和(46.53±3.66)%,对照组分别为(11.99±2.02)%和(31.98±4.22)%,这两个时间点实验组矿化百分比显著高于对照组(P<0.001);术后第5、7、10、14和28天,实验组相较对照组的HIF-1α蛋白相对表达量为2.86±0.48、1.32±0.26、1.32±0.32、1.28±0.38、1.05±0.34,仅术后第5天两组间差异有统计学意义(P<0.01)。结论:在骨缺损局部使用DFO可促进血管化及骨再生,可短暂维持HIF-1α蛋白活性。
中图分类号:
- R318.08
| [1] |
Yellowley CE, Genetos DC. Hypoxia signaling in the skeleton: Implications for bone health[J]. Curr Osteoporos Rep, 2019, 17(1):26-35.
doi: 10.1007/s11914-019-00500-6 pmid: 30725321 |
| [2] |
Holden P, Nair L. Deferoxamine: An angiogenic and antioxidant molecule for tissue regeneration[J]. Tissue Eng Part B Rev, 2019, 25(6):461-470.
doi: 10.1089/ten.teb.2019.0111 |
| [3] |
Temiz G, Sirinoglu H, Yesiloglu N, et al. Effects of deferoxamine on fat graft survival[J]. Facial Plast Surg, 2016, 32(4):438-443.
doi: 10.1055/s-0036-1584236 |
| [4] | Farzan R, Moeinian M, Abdollahi A, et al. Effects of amniotic membrane extract and deferoxamine on angiogenesis in wound healing: An in vivo model[J]. J Wound Care, 2018, 27(Suppl 6):s26-s32. |
| [5] |
Ram M, Singh V, Kumawat S, et al. Deferoxamine modulates cytokines and growth factors to accelerate cutaneous wound healing in diabetic rats[J]. Eur J Pharmacol, 2015, 764:9-21.
doi: 10.1016/j.ejphar.2015.06.029 |
| [6] |
Gao SQ, Chang C, Li JJ, et al. Co-delivery of deferoxamine and hydroxysafflor yellow A to accelerate diabetic wound healing via enhanced angiogenesis[J]. Drug Deliv, 2018, 25(1):1779-1789.
doi: 10.1080/10717544.2018.1513608 |
| [7] |
Yang Q, He GW, Underwood MJ, et al. Cellular and molecular mechanisms of endothelial ischemia/reperfusion injury: Perspectives and implications for postischemic myocardial protection[J]. Am J Transl Res, 2016, 8(2):765-777.
pmid: 27158368 |
| [8] |
Jia P, Chen H, Kang H, et al. Deferoxamine released from poly(lactic-co-glycolic acid) promotes healing of osteoporotic bone defect via enhanced angiogenesis and osteogenesis[J]. J Biomed Mater Res A, 2016, 104(10):2515-2527.
doi: 10.1002/jbm.a.35793 |
| [9] | 姚洋, 杜宇, 古霞, 等. 局部注射外源性神经生长因子促进小鼠钛种植体周骨胶原早期成熟的研究[J]. 华西口腔医学杂志, 2018, 36(2):128-132. |
| [10] |
Koivunen P, Serpi R, Dimova EY. Hypoxia-inducible factor prolyl 4-hydroxylase inhibition in cardiometabolic diseases[J]. Pharmacol Res, 2016, 114:265-273.
doi: S1043-6618(16)31160-4 pmid: 27832958 |
| [11] | Lanigan S, Corcoran AE, Wall A, et al. Acute hypoxic exposure and prolyl-hydroxylase inhibition improves synaptic transmission recovery time from a subsequent hypoxic insult in rat hippocampus[J]. Brain Res, 2018, 1701:212-218. |
| [12] |
Farberg AS, Sarhaddi D, Donneys A, et al. Deferoxamine enhances bone regeneration in mandibular distraction osteogenesis[J]. Plast Reconstr Surg, 2014, 133(3):666-671.
doi: 10.1097/01.prs.0000438050.36881.a9 pmid: 24572857 |
| [13] |
Zhang J, Zheng L, Wang Z, et al. Lowering iron level protects against bone loss in focally irradiated and contralateral femurs through distinct mechanisms[J]. Bone, 2019, 120:50-60.
doi: S8756-3282(18)30369-7 pmid: 30304704 |
| [14] |
Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions[J]. Nat Rev Rheumatol, 2015, 11(1):45-54.
doi: 10.1038/nrrheum.2014.164 pmid: 25266456 |
| [15] | Morgan EF, Giacomo A, Gerstenfeld LC. Overview of skeletal repair (fracture healing and its assessment)[J]. Methods Mol Biol, 2021, 2230:17-37. |
| [16] |
Donneys A, Deshpande SS, Tchanque-Fossuo CN, et al. Deferoxa-mine expedites consolidation during mandibular distraction osteogenesis[J]. Bone, 2013, 55(2):384-390.
doi: 10.1016/j.bone.2013.04.005 pmid: 23598047 |
| [17] | Zimna A, Kurpisz M. Hypoxia-inducible factor-1 in physiological and pathophysiological angiogenesis: Applications and therapies[J]. Biomed Res Int, 2015, 2015:549412. |
| [18] |
Drager J, Harvey EJ, Barralet J. Hypoxia signalling manipulation for bone regeneration[J]. Expert Rev Mol Med, 2015, 17:e6.
doi: 10.1017/erm.2015.4 |
| [19] |
Matsubara H, Hogan DE, Morgan EF, et al. Vascular tissues are a primary source of BMP2 expression during bone formation induced by distraction osteogenesis[J]. Bone, 2012, 51(1):168-180.
doi: 10.1016/j.bone.2012.02.017 pmid: 22391215 |
| [20] |
Bouletreau PJ, Warren SM, Spector JA, et al. Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells: Implications for fracture healing[J]. Plast Reconstr Surg, 2002, 109(7):2384-2397.
pmid: 12045566 |
| [1] | 董佳芸,李雪芬,路瑞芳,胡文杰,孟焕新. 血管化骨瓣重建颌骨种植体周软组织病理学特点[J]. 北京大学学报(医学版), 2024, 56(1): 25-31. |
| [2] | 张胜男,安娜,欧阳翔英,刘颖君,王雪奎. 生长停滞特异性蛋白6在人牙周膜细胞迁移及成骨分化中的作用[J]. 北京大学学报(医学版), 2021, 53(1): 9-15. |
| [3] | 隋华欣,吕培军,王勇,冯驭驰. 低能量激光照射对人脂肪来源干细胞/海藻酸钠/明胶三维生物打印体成骨能力的影响[J]. 北京大学学报(医学版), 2018, 50(5): 868-875. |
| [4] | 刘婧寅,陈飞,葛严军,魏菱,潘韶霞,冯海兰. 选择性激光熔化种植体对早期骨矿化沉积率的影响[J]. 北京大学学报(医学版), 2018, 50(1): 117-122. |
| [5] | 陈飞,潘韶霞,冯海兰. 转化生长因子β1和血管内皮生长因子在浓缩生长因子各层中的分布及含量特点[J]. 北京大学学报(医学版), 2016, 48(5): 860-865. |
| [6] | 秦雪嫣,赵华翔,张倩,陈峰,林久祥. NELL-1: 高效特异的新型生长因子[J]. 北京大学学报(医学版), 2016, 48(2): 380-383. |
| [7] | 葛雯姝,汤祎熳,张晓,刘云松,周永胜. 构建一种可评价脂肪间充质干细胞成骨向分化的荧光素酶报告系统[J]. 北京大学学报(医学版), 2016, 48(1): 170-174. |
| [8] | 宋杨,王晓飞,王宇光,孙玉春,吕培军△. 人脂肪间充质干细胞与生物材料共混物三维打印体的体内成骨[J]. 北京大学学报(医学版), 2016, 48(1): 45-50. |
| [9] | 欧蒙恩, 张晓, 刘云松, 葛严军, 周永胜. 联合应用基质细胞衍生因子1、辛伐他汀及骨胶原支架进行体内异位成骨[J]. 北京大学学报(医学版), 2015, 47(1): 47-51. |
| [10] | 丁茜,张凤秋,马玉实. 淫羊藿苷对人牙周膜细胞骨向分化基因表达的影响[J]. 北京大学学报(医学版), 2013, 45(6): 975-978. |
| [11] | 刘云松*, 吕珑薇*, 周永胜, 马桂娥, 张晓, 范聪, 邵校. 体内成骨过程中人脂肪基质细胞对新骨形成的促进作用[J]. 北京大学学报(医学版), 2012, 44(6): 916-920. |
| [12] | 张驰. 成骨细胞特异性转录因子Osterix对骨形成作用的分子机制[J]. 北京大学学报(医学版), 2012, 44(5): 659-665. |
| [13] | 余日月*, 曾百进. 重组人肿瘤坏死因子α对人脂肪基质细胞体外成骨向分化的影响[J]. 北京大学学报(医学版), 2012, 44(3): 475-480. |
| [14] | 杨玲玲, 邵珲, 原鹏波, 郭晓玥, 张小为, 赵扬玉. 缺氧诱导因子-1α及其靶基因在双胎输血综合征胎盘组织中的表达[J]. 北京大学学报(医学版), 2011, 43(6): 792-797. |
| [15] | 陈俊雅, 廖秦平. 环氧化酶-2在子宫内膜癌中的表达[J]. 北京大学学报(医学版), 2009, 41(6): 657-663. |
|
||