北京大学学报(医学版) ›› 2024, Vol. 56 ›› Issue (5): 763-774. doi: 10.19723/j.issn.1671-167X.2024.05.003
13例先天性双侧输精管缺如不育患者的致病基因突变检测
- 福建省移植生物学重点实验室,福建医科大学福总临床医学院(第九〇〇医院),福州 350025
Detection of pathogenic gene mutations in thirteen cases of congenital bilateral absence of vas deferens infertility patients
Ying TANG, Yongbo ZHANG, Danhong WU, Yanhong LIN, Fenghua LAN*( )
)
- Fujian Provincial Key Laboratory of Transplant Biology, Fuzong Clinical Medical College of Fujian Medical University (The 900th Hospital of Joint Logistic Support Force, PLA), Fuzhou 350025, China
摘要:
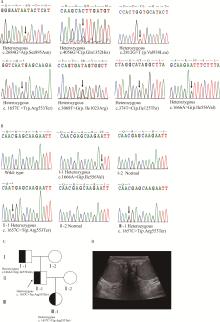
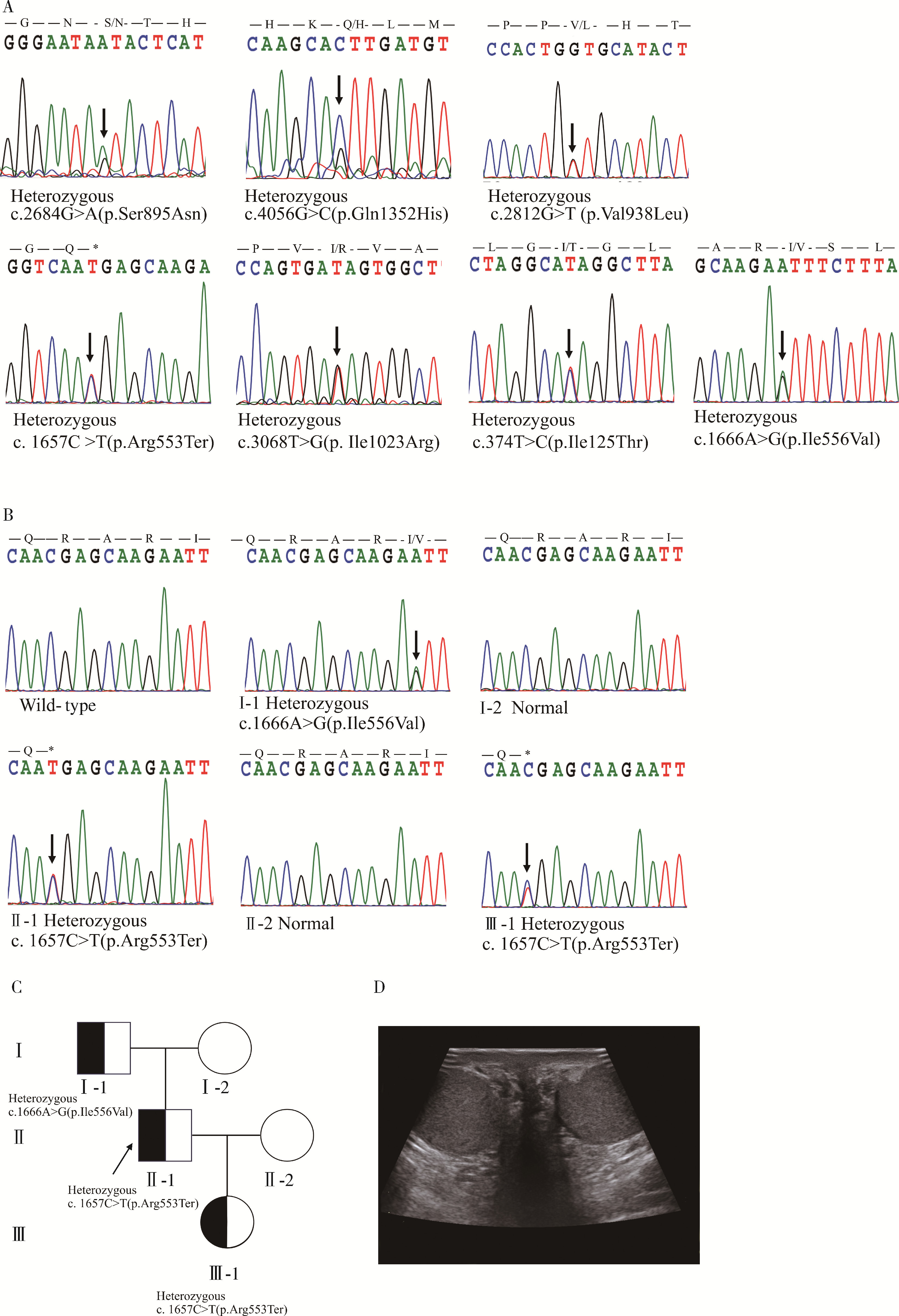
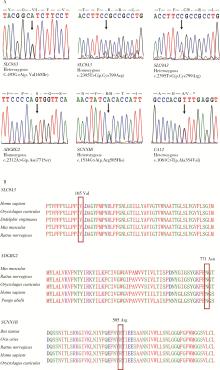
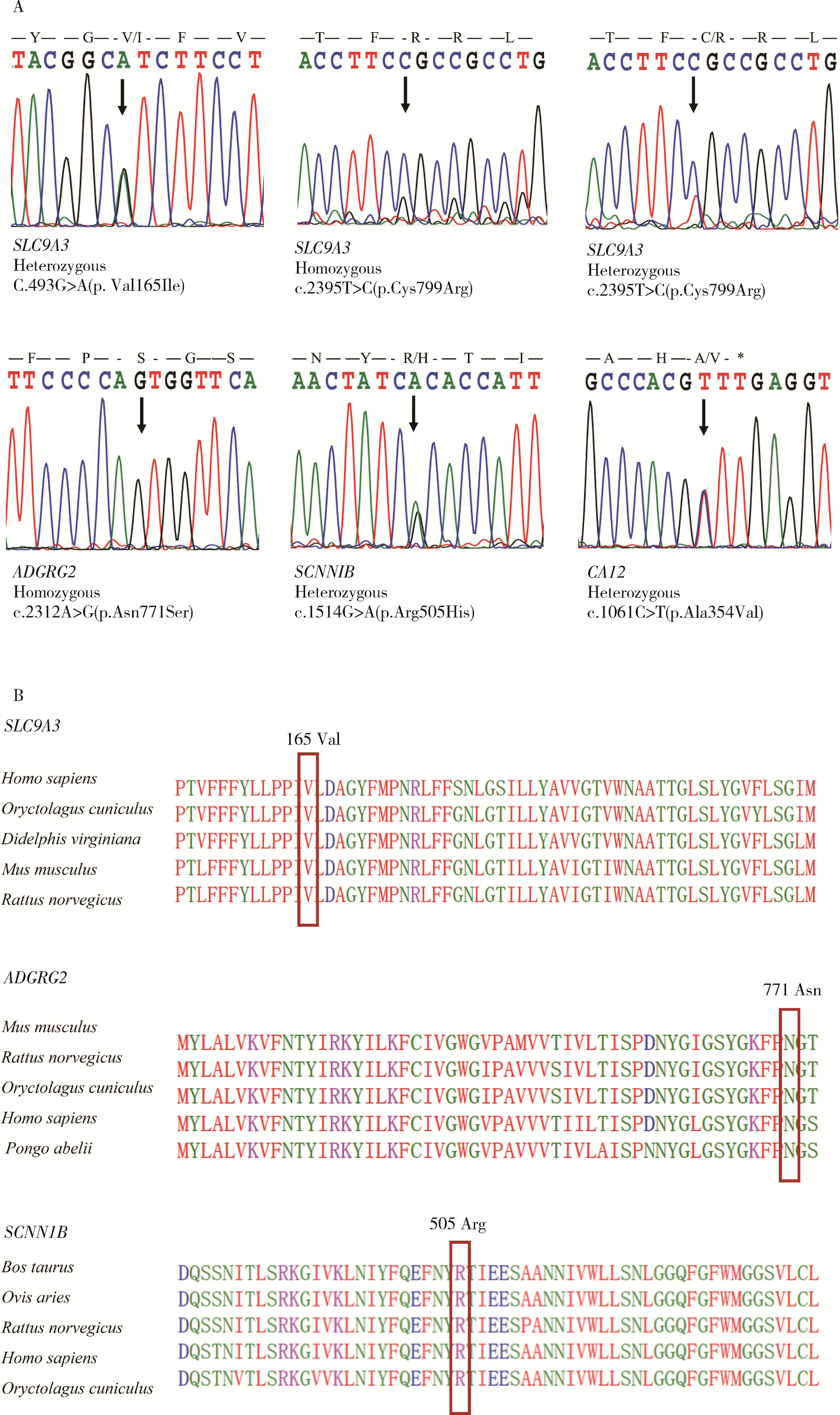
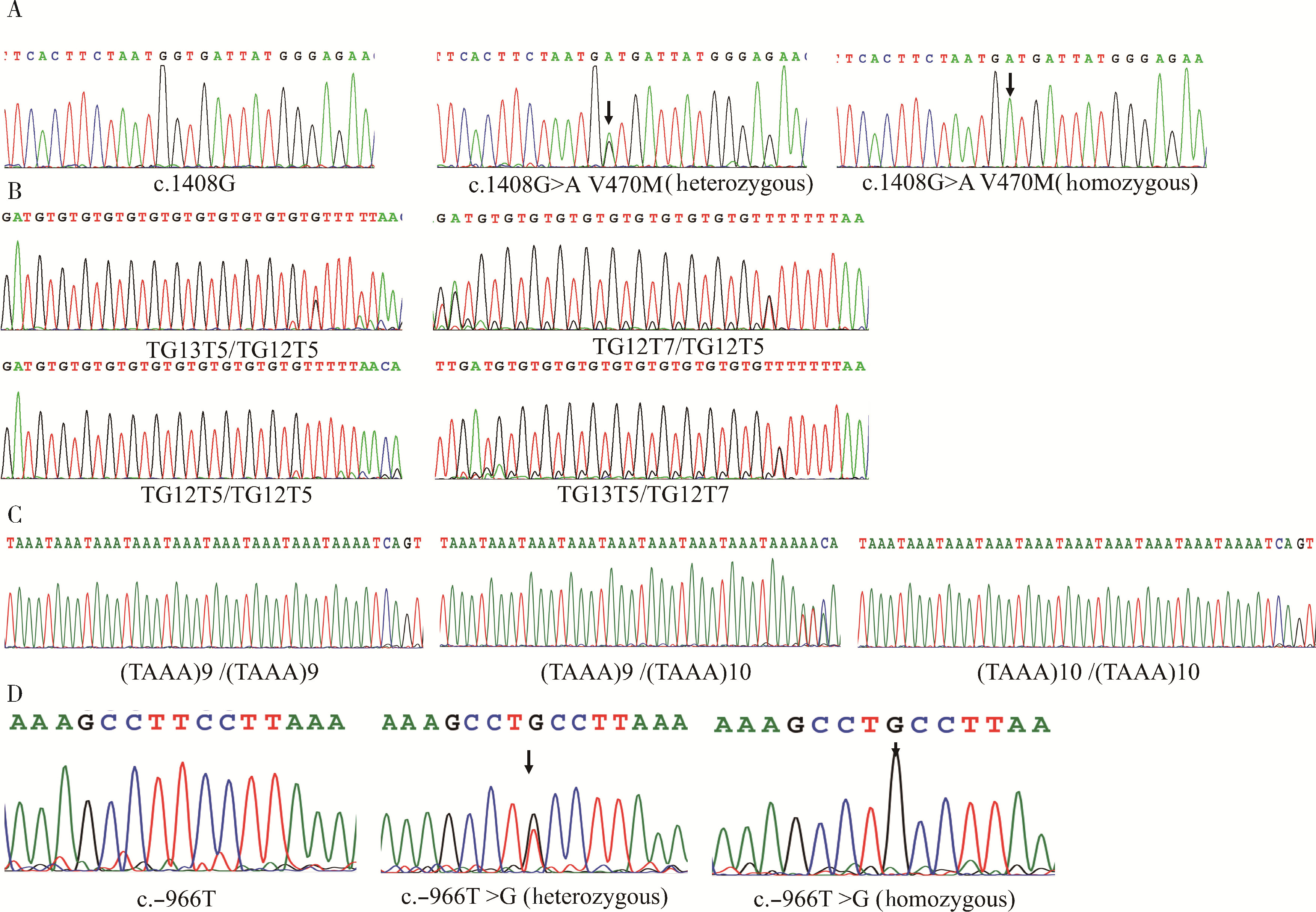
目的: 检测先天性双侧输精管缺如(congenital bilateral absence of the vas deferens, CBAVD)患者中囊性纤维化跨膜转导调节因子(cystic fibrosis transmembrane conductance regulator, CFTR)基因和CBAVD易感基因的突变情况,探讨它们与CBAVD发病风险的相关性。方法: 对13例诊断为孤立发生的CBAVD患者的致病基因CFTR及易感基因黏附型G蛋白偶联受体G2(adhesion G protein-coupled receptor G2, ADGRG2)、上皮细胞钠离子通道β亚单位(sodium channel epithelial 1 subunit beta, SCNN1B)和碳酸酐酶12(carbonic anhydrase, CA12)和溶质载体家族9成员3(solute carrier family 9 member A3, SLC9A3)行全外显子测序及Sanger测序验证,针对CFTR基因多态性位点、内含子及侧翼序列行聚合酶链式反应(polymerase chain reaction, PCR)扩增后用Sanger测序,并运用生物信息学方法对CBAVD易感基因新发突变进行保守性分析和有害性预测。对13例CBAVD患者中1例患者的家系进行遗传学分析,评估子代遗传风险。结果: 外显子测序发现13例CBAVD患者中,只有6例患者检测到CFTR基因外显子突变,有6种错义突变: c.2684G>A(p.Ser895Asn)、c.4056G>C(p.Gln1352His)、c.2812G>T(p.Val938Leu)、c.3068T>G(p.Ile1023Arg)、c.374T>C(p.Ile125Thr)、c.1666A>G(p.Ile556Val), 1种无义突变: c.1657C>T(p.Arg553Ter), 这6例患者中有2例患者同时还存在CFTR的纯合p.V470位点,另外7例患者未检测出CFTR基因外显子区域的突变。13例CBAVD患者中,3例患者携带纯合p.V470的多态性位点,4例患者携带5T等位基因,2例患者携带TG13等位基因,10例患者携带c.-966T>G位点。4例CBAVD患者同时携带以上CFTR基因突变位点中的2~3个位点。13例患者中CBAVD易感基因突变情况: 1种ADGRG2错义突变c.2312A>G(p.Asn771Ser),2种SLC9A3错义突变c.2395T>C(p.Cys799Arg)、c.493G>A(p.Val165Ile), 1种SCNN1B错义突变c.1514G>A(p.Arg505His)和1种CA12错义突变c.1061C>T(p.Ala354Val), 其中,SLC9A3基因的c.493G>A(p.Val165Ile)突变位点是首次在CBAVD患者中被发现,以上5种突变位点在gnomAD数据库中的人群变异频率极低,属于罕见突变,用Mutation Taster和Polyphen-2软件预测显示SLC9A3基因的c.493G>A(p.Val165Ile)位点和SCNN1B基因的c.1514G>A(p.Arg505His)位点的有害性等级为致病突变。1例家系遗传分析发现,先证者的c.1657C>T(p.Arg553Ter)突变为新生突变,先证者父亲、母亲均未携带该突变,先证者及其配偶通过辅助生殖技术孕育1女婴,该女婴遗传了先证者的致病性突变c.1657C>T(p.Arg553Ter)。结论: CFTR基因突变仍然是中国CBAVD患者的主要致病原因,但突变的分布与频率与国内外其他研究的数据存在一定差异,需要进一步扩充中国CBAVD患者的CFTR突变谱; ADGRG2、SLC9A3、SCNN1B和CA12易感基因可能解释部分无CFTR突变的CBAVD病例; CBAVD患者多无特殊临床表现,建议临床医生确诊前对患者行进一步的体格检查,并结合其阴囊超声或经直肠超声检查; 建议将CFTR基因突变检测应用于辅助生殖前的遗传学筛查,降低子代罹患CBAVD及囊性纤维化的风险。
中图分类号:
- R394.1
| 1 |
Yang B , Lei C , Yang D , et al. Whole-exome sequencing identified CFTR variants in two consanguineous families in China[J]. Front Genet, 2021, 12, 631221.
doi: 10.3389/fgene.2021.631221 |
| 2 |
王宇刚, 马淑霞, 姚晓飞, 等. 男性不育伴精液量少的临床特点分析[J]. 生殖医学杂志, 2023, 32 (7): 997- 1002.
doi: 10.3969/j.issn.1004-3845.2023.07.005 |
| 3 |
Chillón M , Casals T , Mercier B , et al. Mutations in the cystic fibrosis gene in patients with congenital absence of the vas deferens[J]. N Engl J Med, 1995, 332 (22): 1475- 1480.
doi: 10.1056/NEJM199506013322204 |
| 4 |
Qu X , Li L , Cui C , et al. Correlation between CFTR variants and outcomes of ART in patients with CAVD in Central China[J]. Sci Rep, 2023, 13 (1): 64.
doi: 10.1038/s41598-022-26384-8 |
| 5 |
Wang H , An M , Liu Y , et al. Genetic diagnosis and sperm retrieval outcomes for Chinese patients with congenital bilateral absence of vas deferens[J]. Andrology, 2020, 8 (5): 1064- 1069.
doi: 10.1111/andr.12769 |
| 6 |
Fang J , Wang X , Sun X , et al. Congenital absence of the vas deferens with hypospadias or without hypospadias: Phenotypic findings and genetic considerations[J]. Front Genet, 2022, 13, 1035468.
doi: 10.3389/fgene.2022.1035468 |
| 7 |
Bieth E , Hamdi SM , Mieusset R . Genetics of the congenital absence of the vas deferens[J]. Hum Genet, 2021, 140 (1): 59- 76.
doi: 10.1007/s00439-020-02122-w |
| 8 |
Lu Y , Xie Y , Li M , et al. A novel ADGRG2 truncating variant associated with X-linked obstructive azoospermia in a large Chinese pedigree[J]. J Assist Reprod Genet, 2023, 40 (7): 1747- 1754.
doi: 10.1007/s10815-023-02839-3 |
| 9 | Wu YN , Chen KC , Wu CC , et al. SLC9A3 affects vas deferens development and associates with Taiwanese congenital bilateral absence of the vas deferens[J]. Biomed Res Int, 2019, 10, 3562719. |
| 10 |
Shen Y , Yue HX , Li FP , et al. SCNN1B and CA12 play vital roles in occurrence of congenital bilateral absence of vas deferens (CBAVD)[J]. Asian J Androl, 2019, 21 (5): 525- 527.
doi: 10.4103/aja.aja_112_18 |
| 11 |
Guo X , Liu K , Liu Y , et al. Clinical and genetic characteristics of cystic fibrosis in Chinese patients: A systemic review of reported cases[J]. Orphanet J Rare Dis, 2018, 13 (1): 224.
doi: 10.1186/s13023-018-0968-2 |
| 12 |
Souza PFN , Amaral JL , Bezerra LP , et al. ACE2-derived peptides interact with the RBD domain of SARS-CoV-2 spike glycoprotein, disrupting the interaction with the human ACE2 receptor[J]. J Biomol Struct Dyn, 2022, 40 (12): 5493- 5506.
doi: 10.1080/07391102.2020.1871415 |
| 13 | 顾怡栋, 邓学东, 程洪波, 等. CBAVD患者超声分型在穿刺取精方式选择中的应用价值[J]. 医学影像学杂志, 2019, 29 (9): 1621- 1625. |
| 14 |
Prins S , Corradi V , Sheppard DN , et al. Can two wrongs make a right? F508del-CFTR ion channel rescue by second-site mutations in its transmembrane domains[J]. J Biol Chem, 2022, 298, 101615.
doi: 10.1016/j.jbc.2022.101615 |
| 15 |
Gaikwad A , Khan S , Kadam S , et al. Cystic fibrosis transmembrane conductance regulator-related male infertility: Relevance of genetic testing & counselling in Indian population[J]. Indian J Med Res, 2020, 152 (6): 575- 583.
doi: 10.4103/ijmr.IJMR_906_18 |
| 16 | Li H , Lin L , Hu X , et al. Liver failure in a Chinese cystic fibrosis child with homozygous R553X mutation[J]. Front Pediatr, 2019, 20 (7): 36. |
| 17 |
Leung GKC , Ying D , Mak CCY , et al. CFTR founder mutation causes protein trafficking defects in Chinese patients with cystic fibrosis[J]. Mol Genet Genomic Med, 2017, 5 (1): 40- 49.
doi: 10.1002/mgg3.258 |
| 18 |
Luo S , Feng J , Zhang Y , et al. Mutation analysis of the cystic fibrosis transmembrane conductance regulator gene in Chinese congenital absence of vas deferens patients[J]. Gene, 2021, 765, 145045.
doi: 10.1016/j.gene.2020.145045 |
| 19 |
Tadaka S , Hishinuma E , Komaki S , et al. jMorp updates in 2020:Large enhancement of multi-omics data resources on the general Japanese population[J]. Nucleic Acids Res, 2021, 49 (D1): D536- D544.
doi: 10.1093/nar/gkaa1034 |
| 20 |
Le VS , Tran KT , Bui H TP , et al. A Vietnamese human genetic variation database[J]. Hum Mutat, 2019, 40 (10): 1664- 1675.
doi: 10.1002/humu.23835 |
| 21 |
Gaikwad A , Khan S , Kadam S , et al. The CFTR gene mild variants poly-T, TG repeats and M470V detection in Indian men with congenital bilateral absence of vas deferens[J]. Andrologia, 2018, 50 (2): e12858.
doi: 10.1111/and.12858 |
| 22 |
Casals T , Bassas L , Egozcue S , et al. Heterogeneity for mutations in the CFTR gene and clinical correlations in patients with con-genital absence of the vas deferens[J]. Hum Reprod, 2000, 15 (7): 1476- 1483.
doi: 10.1093/humrep/15.7.1476 |
| 23 |
Grangeia A , Niel F , Carvalho F , et al. Characterization of cystic fibrosis conductance transmembrane regulator gene mutations and IVS8 poly(T) variants in Portuguese patients with congenital absence of the vas deferens[J]. Hum Reprod, 2004, 19 (11): 2502- 2508.
doi: 10.1093/humrep/deh462 |
| 24 |
Fathy M , Ramzy T , Elmonem MA , et al. Molecular screening of CFTR gene in Egyptian patients with congenital bilateral absence of the vas deferens: A preliminary study[J]. Andrologia, 2016, 48 (10): 1307- 1312.
doi: 10.1111/and.12563 |
| 25 |
Cheng H , Yang S , Meng Q , et al. Genetic analysis and intracytoplasmic sperm injection outcomes of Chinese patients with congenital bilateral absence of vas deferens[J]. J Assist Reprod Genet, 2022, 39 (3): 719- 728.
doi: 10.1007/s10815-022-02417-z |
| 26 |
Yuan P , Liang ZK , Liang H , et al. Expanding the phenotypic and genetic spectrum of Chinese patients with congenital absence of vas deferens bearing CFTR and ADGRG2 alleles[J]. Andrology, 2019, 7 (3): 329- 340.
doi: 10.1111/andr.12592 |
| 27 |
Ni WH , Jiang L , Fei QJ , et al. The CFTR polymorphisms poly-T, TG-repeats and M470V in Chinese males with congenital bila-teral absence of the vas deferens[J]. Asian J Androl, 2012, 14 (5): 687- 690.
doi: 10.1038/aja.2012.43 |
| 28 |
Tosco A , Castaldo A , Colombo C , et al. Clinical outcomes of a large cohort of individuals with the F508del/5T; TG12 CFTR ge-notype[J]. J Cyst Fibros, 2022, 21 (5): 850- 855.
doi: 10.1016/j.jcf.2022.04.020 |
| 29 |
Cuppens H , Lin W , Jaspers M , et al. Polyvariant mutant cystic fibrosis transmembrane conductance regulator genes. The polymorphic (Tg)m locus explains the partial penetrance of the T5 polymorphism as a disease mutation[J]. J Clin Invest, 1998, 101 (2): 487- 496.
doi: 10.1172/JCI639 |
| 30 |
Nykamp K , Truty R , Riethmaier D , et al. Elucidating clinical phenotypic variability associated with the polyT tract and TG repeats in CFTR[J]. Hum Mutat, 2021, 42 (9): 1165- 1172.
doi: 10.1002/humu.24250 |
| 31 |
Kerschner JL , Meckler F , Coatti GC , et al. The impact of geno-mic distance on enhancer-promoter interactions at the CFTR locus[J]. J Cell Mol Med, 2024, 28 (4): e18142.
doi: 10.1111/jcmm.18142 |
| 32 |
Verlingue C , Vuillaumier S , Mercier B , et al. Absence of mutations in the interspecies conserved regions of the CFTR promoter region in cystic fibrosis (CF) and CF related patients[J]. J Med Genet, 1998, 35 (2): 137- 140.
doi: 10.1136/jmg.35.2.137 |
| 33 | Bai S , Du Q , Liu X , et al. The detection and significance of cys-tic fibrosis transmembrane conductance regulator gene promoter mutations in Chinese patients with congenital bilateral absence of the vas deferens[J]. Gene, 2018, 672 (25): 64- 71. |
| 34 |
Zhuo JL , Soleimani M , Li XC . New insights into the critical importance of intratubular Na(+)/H(+) exchanger 3 and its potential therapeutic implications in hypertension[J]. Curr Hypertens Rep, 2021, 23 (6): 34.
doi: 10.1007/s11906-021-01152-7 |
| 35 |
Wang YY , Lin YH , Wu YN , et al. Loss of SLC9A3 decreases CFTR protein and causes obstructed azoospermia in mice[J]. PLoS Genet, 2017, 13 (4): e1006715.
doi: 10.1371/journal.pgen.1006715 |
| 36 |
Karacan Küçükali G , çetinkaya S , Tunç G , et al. Clinical management in systemic type pseudohypoaldosteronism due to SCNN1B variant and literature review[J]. J Clin Res Pediatr Endocrinol, 2021, 13 (4): 446- 451.
doi: 10.4274/jcrpe.galenos.2020.2020.0107 |
| 37 |
Ideozu JE , Liu M , Riley-Gillis BM , et al. Diversity of CFTR variants across ancestries characterized using 454 727 UK biobank whole exome sequences[J]. Genome Med, 2024, 16 (1): 43.
doi: 10.1186/s13073-024-01316-5 |
| 38 |
Ni Q , Chen X , Zhang P , et al. Systematic estimation of cystic fibrosis prevalence in Chinese and genetic spectrum comparison to Caucasians[J]. Orphanet J Rare Dis, 2022, 17 (1): 129.
doi: 10.1186/s13023-022-02279-9 |
| [1] | 薛恩慈, 陈曦, 王雪珩, 王斯悦, 王梦莹, 李劲, 秦雪英, 武轶群, 李楠, 李静, 周治波, 朱洪平, 吴涛, 陈大方, 胡永华. 中国人群非综合征型唇裂伴或不伴腭裂的单核苷酸多态性遗传度[J]. 北京大学学报(医学版), 2024, 56(5): 775-780. |
| [2] | 吴君怡,余淼,孙仕晨,樊壮壮,郑静蕾,张刘陶,冯海兰,刘洋,韩冬. 少汗性外胚层发育不良患者EDA基因突变检测及表型分析[J]. 北京大学学报(医学版), 2021, 53(1): 24-33. |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 77
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 165
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
Cited |
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| Shared | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Discussed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
||