北京大学学报(医学版) ›› 2025, Vol. 57 ›› Issue (4): 764-771. doi: 10.19723/j.issn.1671-167X.2025.04.022
基于秀丽线虫模型探究七叶皂苷和右美沙芬对阿尔茨海默病的保护作用
张一平1, 李璐迪1, 朱安2, 肖武生1, 王旗1,*( )
)
- 1. 北京大学公共卫生学院毒理学系,国家中医药管理局中药配伍减毒重点研究室,食品安全毒理学研究与评价北京市重点实验室,北京 100191
2. 福建医科大学基础医学院消化道恶性肿瘤教育部重点实验室,福州 350108
Protective effects of escin and dextromethorphan on Alzheimer disease in Caenorhabditis elegans models
Yiping ZHANG1, Ludi LI1, An ZHU2, Wusheng XIAO1, Qi WANG1,*( )
)
- 1. Department of Toxicology, Peking University School of Public Health, Key Laboratory of State Administration of Traditional Chinese Medicine for Compatibility Toxicology, Beijing Key Laboratory of Toxicological Research and Risk Assessment for Food Safety, Beijing 100191, China
2. Key Laboratory of Gastrointestinal Malignant Tumors, Basic Medical College, Fujian Medical University, Ministry of Education, Fuzhou 350108, China
摘要:
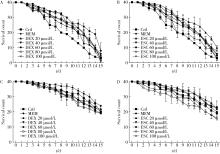
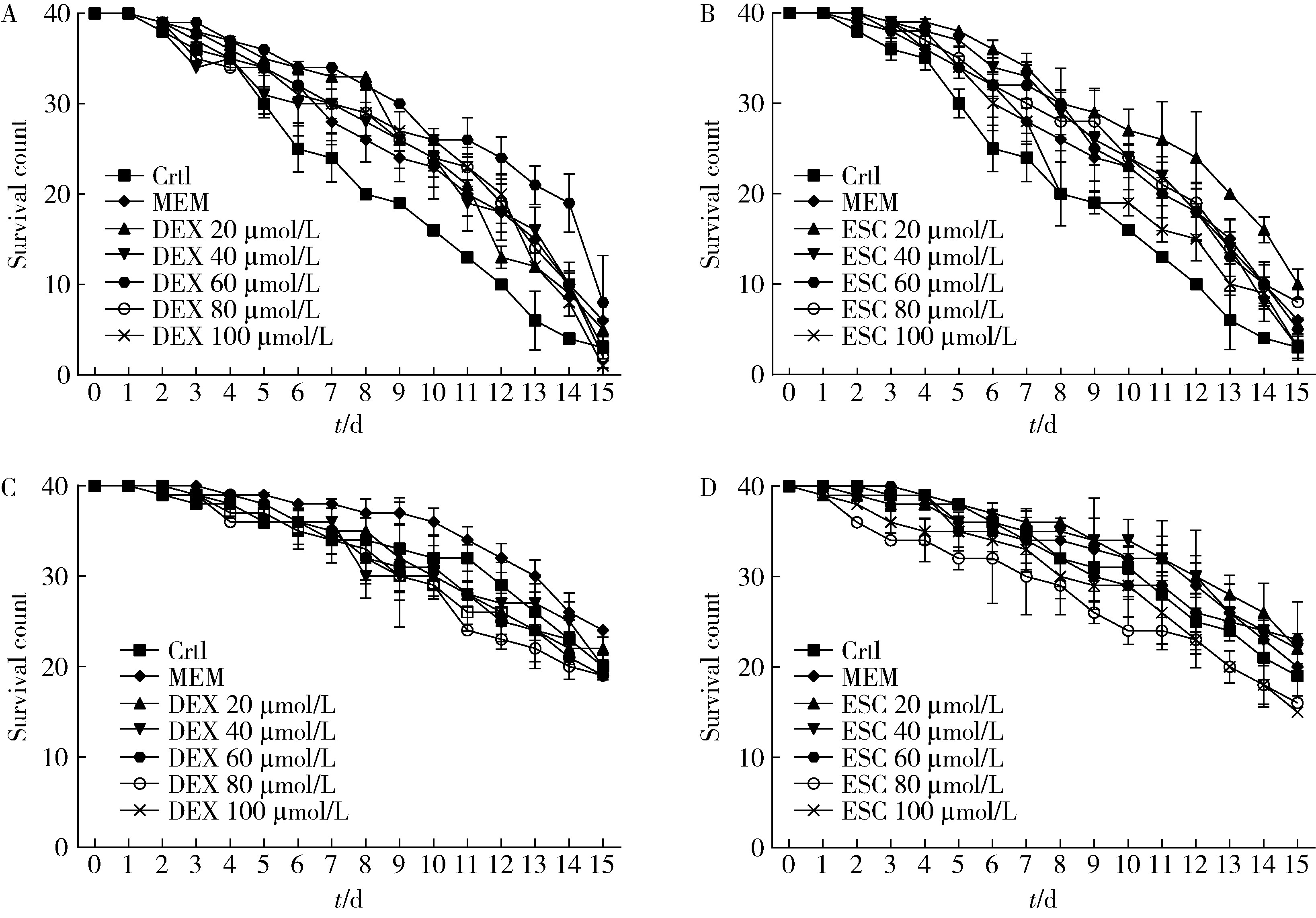
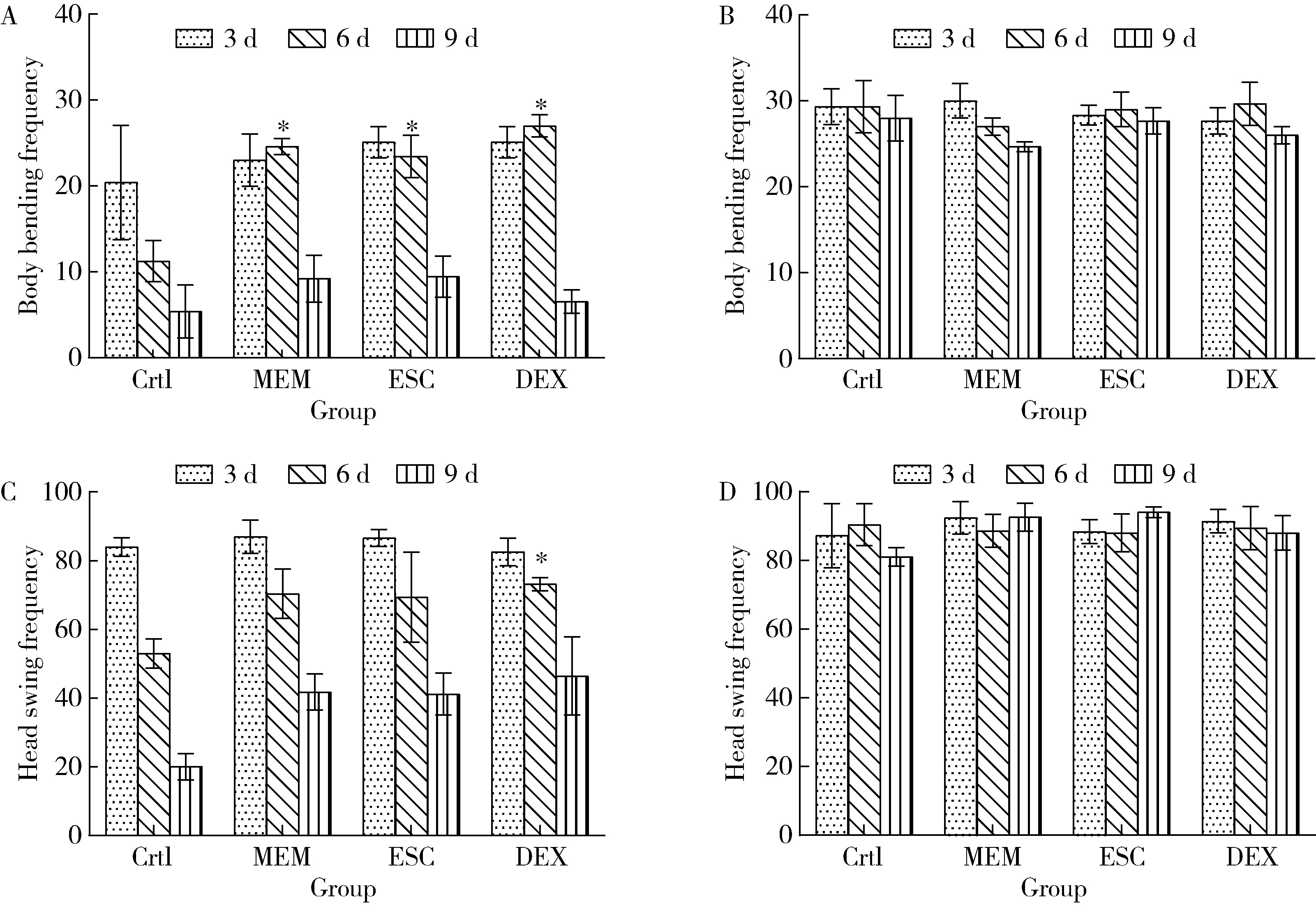
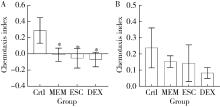
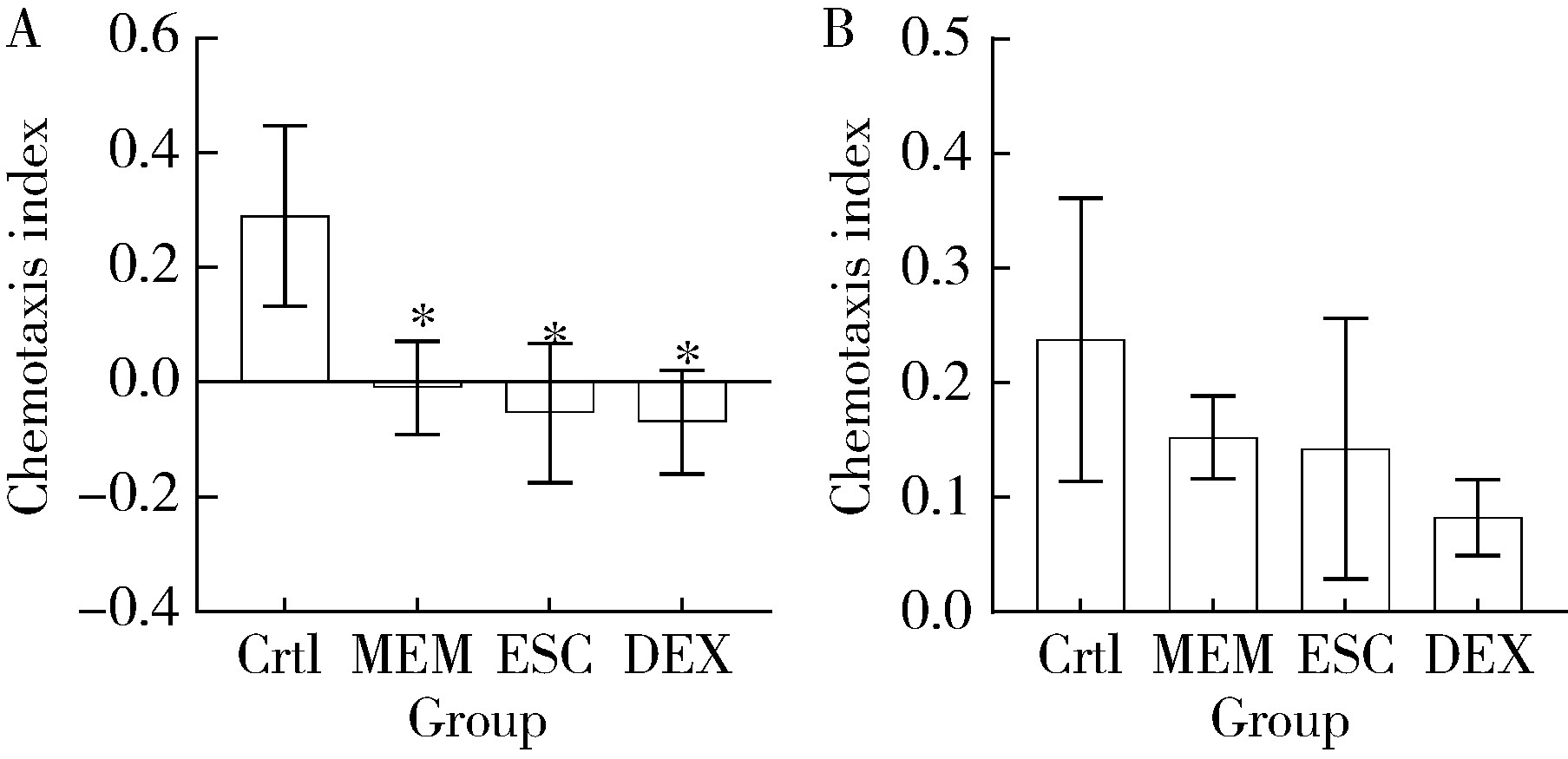
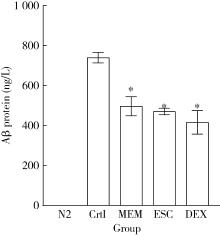
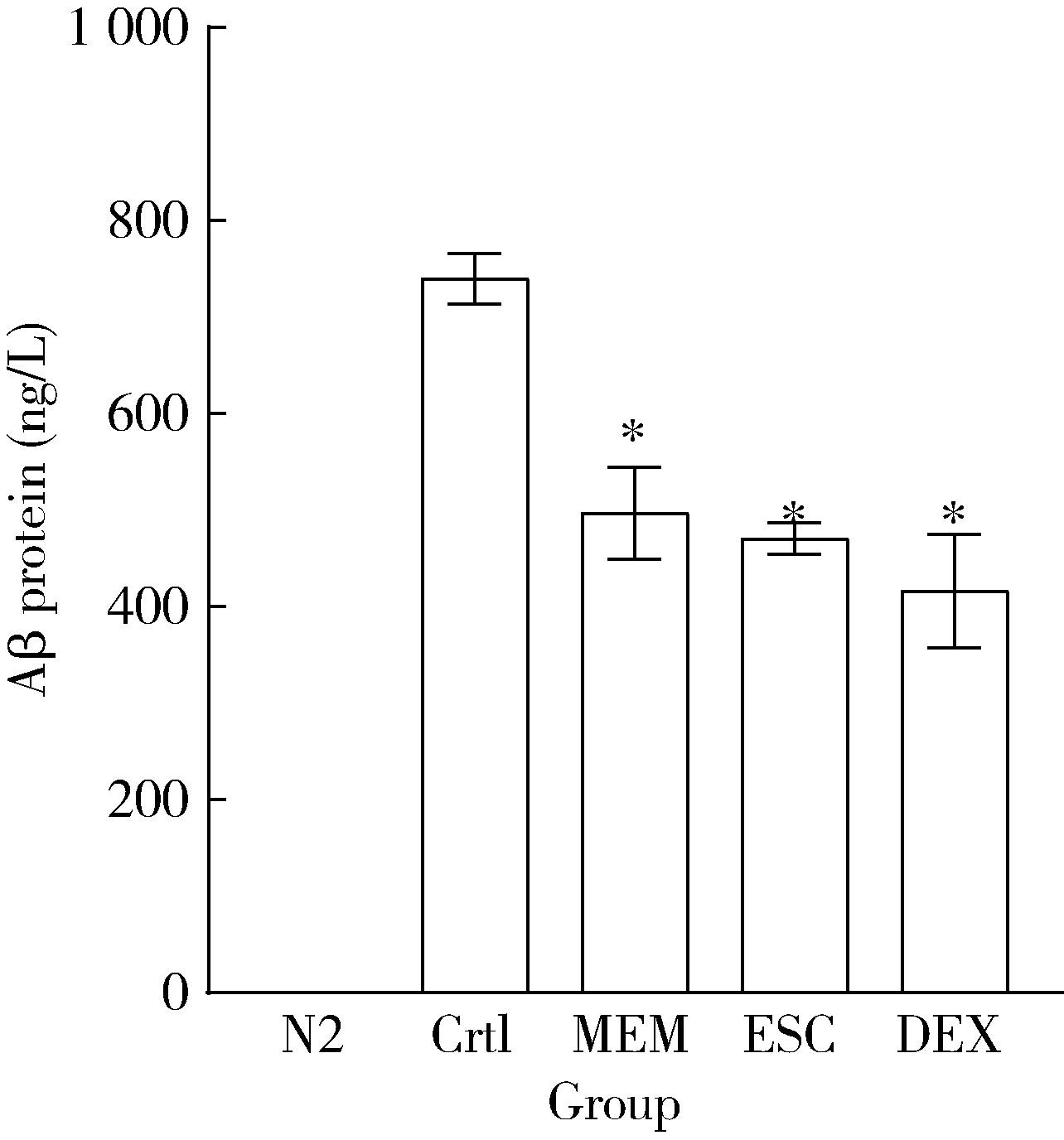
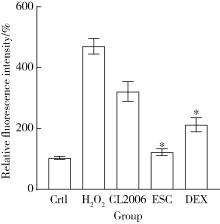
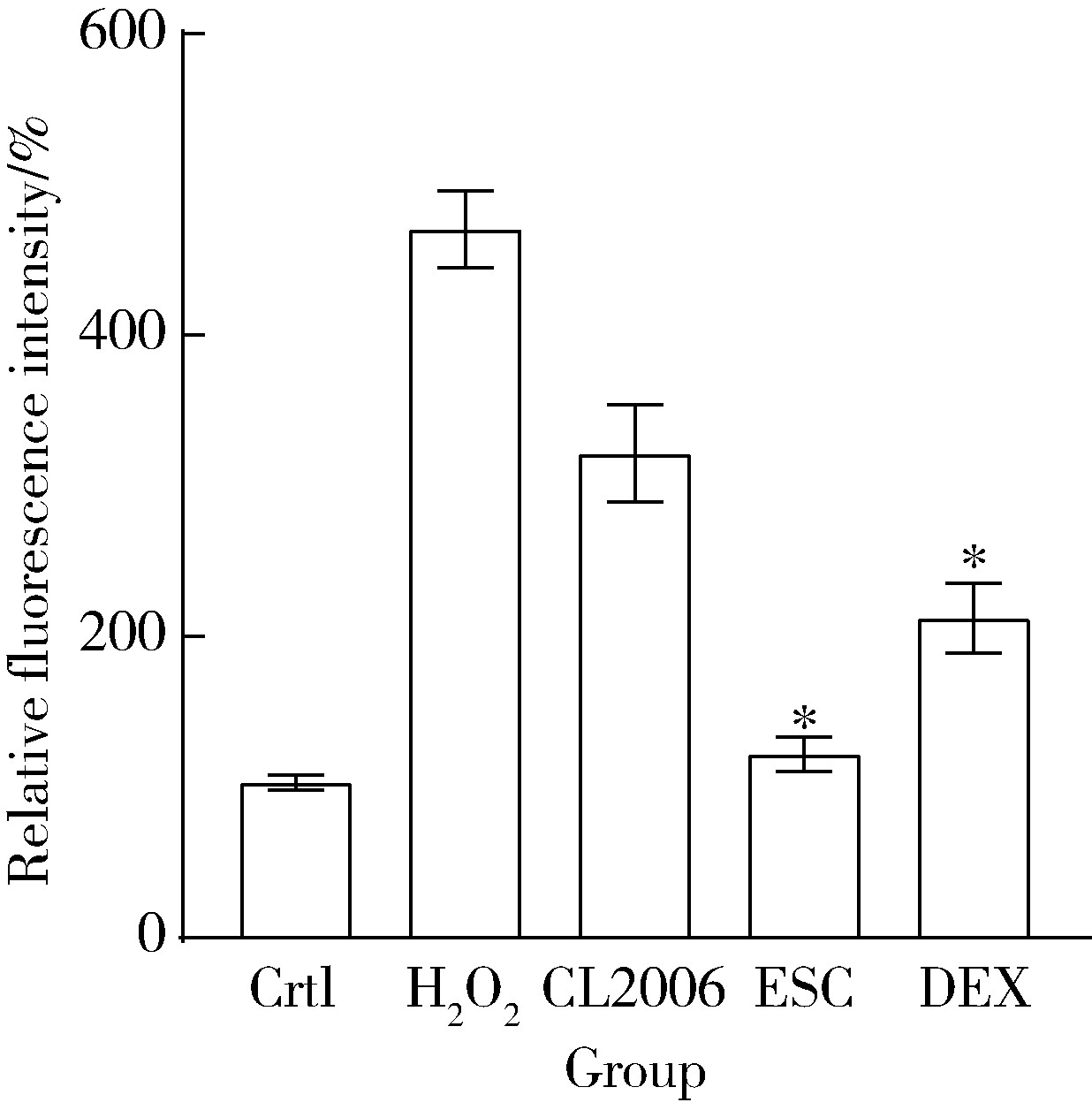
目的: 探究七叶皂苷(escin,ESC)和右美沙芬(dextromethorphan,DEX)是否具有延缓阿尔茨海默病(Alzheimer disease,AD)的作用。方法: 使用秀丽隐杆线虫β-淀粉样蛋白(amyloid β-protein,Aβ)转基因构建的AD模型,采用固体暴露方式,给予不同浓度的ESC和DEX处理,以美金刚(memantine,MEM)50 μmol/L作为阳性对照组,检测线虫的寿命变化、运动能力和认知功能变化、Aβ蛋白表达量,以及活性氧含量。通过实时荧光定量PCR检测氧化应激通路相关基因的表达。结果: 高剂量处理(1 000 μmol/L ESC或DEX)对野生型N2线虫活动无明显影响。与空白对照组相比,20 μmol/L ESC组和60 μmo/L DEX组显著延长AD模型线虫的生存时间。在AD发病中期,ESC和DEX可减少AD模型线虫的身体弯曲频率的降低,且DEX能明显改善AD模型线虫的头部摆动频率降低。早期的认知功能测试的趋化指数ESC组和DEX组及阳性对照组显著高于空白对照组,这与其体内Aβ蛋白的含量降低相关。ESC组和DEX组的活性氧含量相较于空白对照组有所减少;且基因表达结果显示,ESC可通过上调抗氧化应激基因skn1的表达来减轻AD模型线虫体内的氧化损伤。结论: ESC和DEX能改善AD模型线虫运动能力和认知功能的降低,延缓AD相关症状的加重。ESC可能通过激活SKN-1/Nrf2通路,降低AD模型线虫的氧化应激进而延缓AD进展。
中图分类号:
- R749.16
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| 5 |
周小涛, 杨梦艳, 张继川. 细胞自噬与阿尔茨海默病的关系[J]. 中国组织化学与细胞化学杂志, 2022, 31 (5): 517- 522.
|
| 6 |
陈长军, 徐为华, 张梦晨, 等. 七叶皂苷药理活性及制剂临床应用研究进展[J]. 武汉大学学报(医学版), 2020, 41 (1): 157- 163.
|
| 7 |
|
| 8 |
徐效凤. 右美沙芬在血管性痴呆中的保护作用及机制研究[D], 上海: 上海交通大学, 2015.
|
| 9 |
|
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
|
| 14 |
|
| 15 |
|
| 16 |
|
| 17 |
|
| 18 |
|
| 19 |
李品洁. 线虫头部运动: 定量分析和神经机制研究[D], 合肥: 中国科学技术大学, 2022.
|
| 20 |
|
| 21 |
|
| 22 |
徐玉兰, 薛雅丹, 康利军. 秀丽隐杆线虫神经胶质细胞对神经系统发育和功能的影响[J]. 浙江大学学报(医学版), 2016, 45 (3): 315- 322.
|
| 23 |
|
| 24 |
|
| 25 |
邹羽真, 韩菲, 梅丹. 右美沙芬在神经系统应用的研究新进展[J]. 中国医院药学杂志, 2019, 39 (3): 313- 317.
|
| [1] | 何丽杰,张春艳,王静. NEAT1、miR-27a-3p在阿尔茨海默病患者血清和脑脊液中的表达关系[J]. 北京大学学报(医学版), 2024, 56(2): 207-212. |
| [2] | 韩济生. 疼痛、药物成瘾和神经退行性疾病最新研究进展[J]. 北京大学学报(医学版), 2009, 41(3): 249-254. |
| [3] | 查芹芹, 阮燕, 刘中华, 曹连元, 张岱. β-淀粉样前体蛋白第二、三外显子删除突变抑制β-淀粉样蛋白分泌[J]. 北京大学学报(医学版), 2003, 35(3): 261-265. |
| [4] | 丁吉新, 阮燕, 朱忠军, 张岱. β-淀粉样蛋白前体N端神经节苷脂GM1结合位点定位分析[J]. 北京大学学报(医学版), 2002, 34(5): 594-598. |
| [5] | 常磊, 马康涛, 张蘅. 阿尔茨海默病淀粉样前体蛋白可与低密度脂蛋白受体相关蛋白6直接作用[J]. 北京大学学报(医学版), 2000, 32(4): 366-368. |
|
||