北京大学学报(医学版) ›› 2026, Vol. 58 ›› Issue (1): 175-183. doi: 10.19723/j.issn.1671-167X.2026.01.023
黑色素瘤缺乏因子2介导的细胞焦亡通路在特发性炎性肌病患者外周血单个核细胞中的表达
初吉燕1,2, 李萍1,*( ), 田竞3, 付笛语1,2, 郭琳1, 孙蕊1, 李亚娣1
), 田竞3, 付笛语1,2, 郭琳1, 孙蕊1, 李亚娣1
- 1. 中国人民解放军北部战区总医院风湿免疫科, 沈阳 110001
2. 大连医科大学研究生院, 辽宁大连 116044
3. 中国人民解放军北部战区总医院骨科, 沈阳 110001
Expression of the melanoma 2-mediated pyroptosis pathway in peripheral blood mononuclear cells of patients with idiopathic inflammatory myopathies
Jiyan CHU1,2, Ping LI1,*( ), Jing TIAN3, Diyu FU1,2, Lin GUO1, Rui SUN1, Yadi LI1
), Jing TIAN3, Diyu FU1,2, Lin GUO1, Rui SUN1, Yadi LI1
- 1. Department of Rheumatology and Immunology, General Hospital of Northern Theater Command, Shenyang 110001, China
2. Graduate School, Dalian Medical University, Dalian 116044, Liaoning, China
3. Department of Orthopedics, General Hospital of Northern Theater Command, Shenyang 110001, China
摘要:
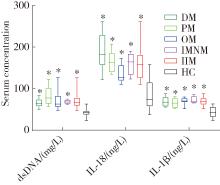
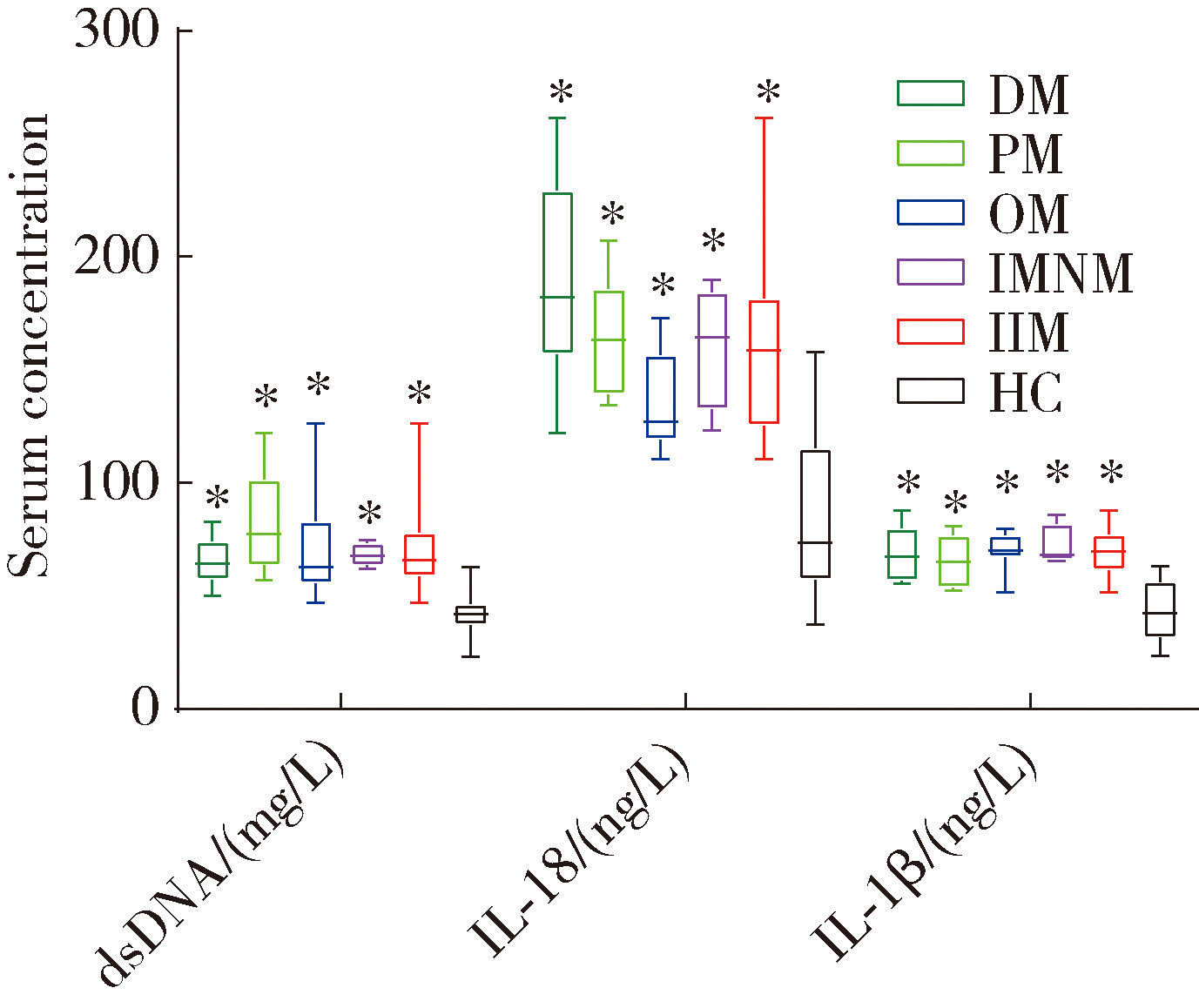
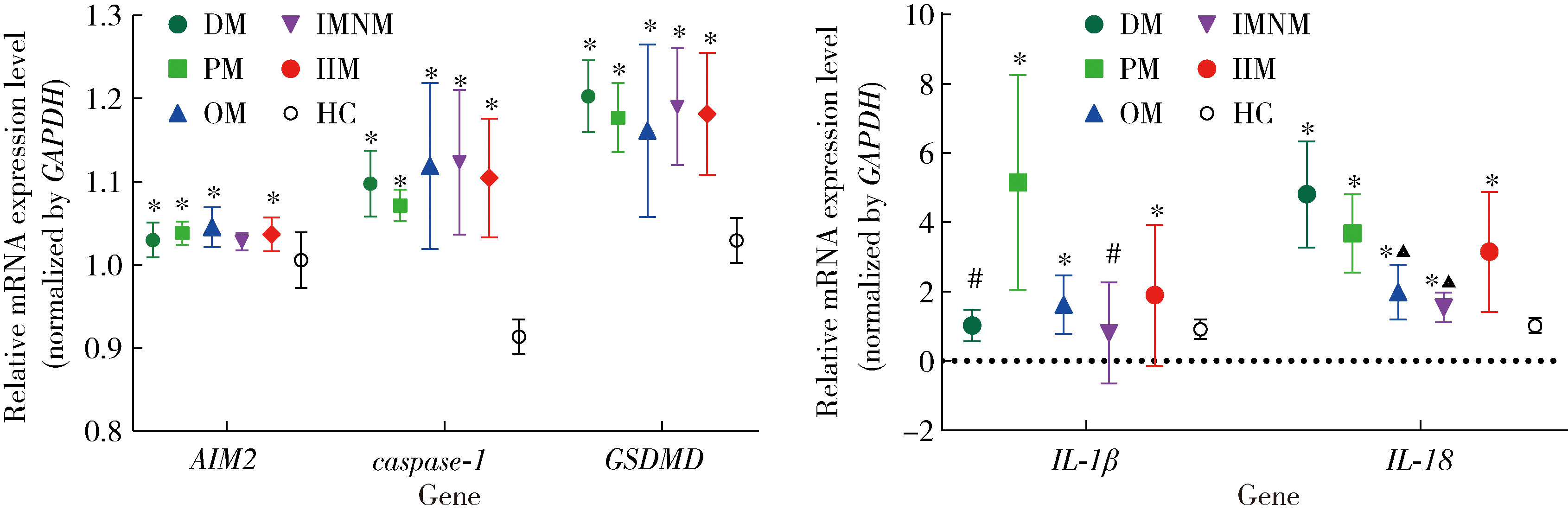
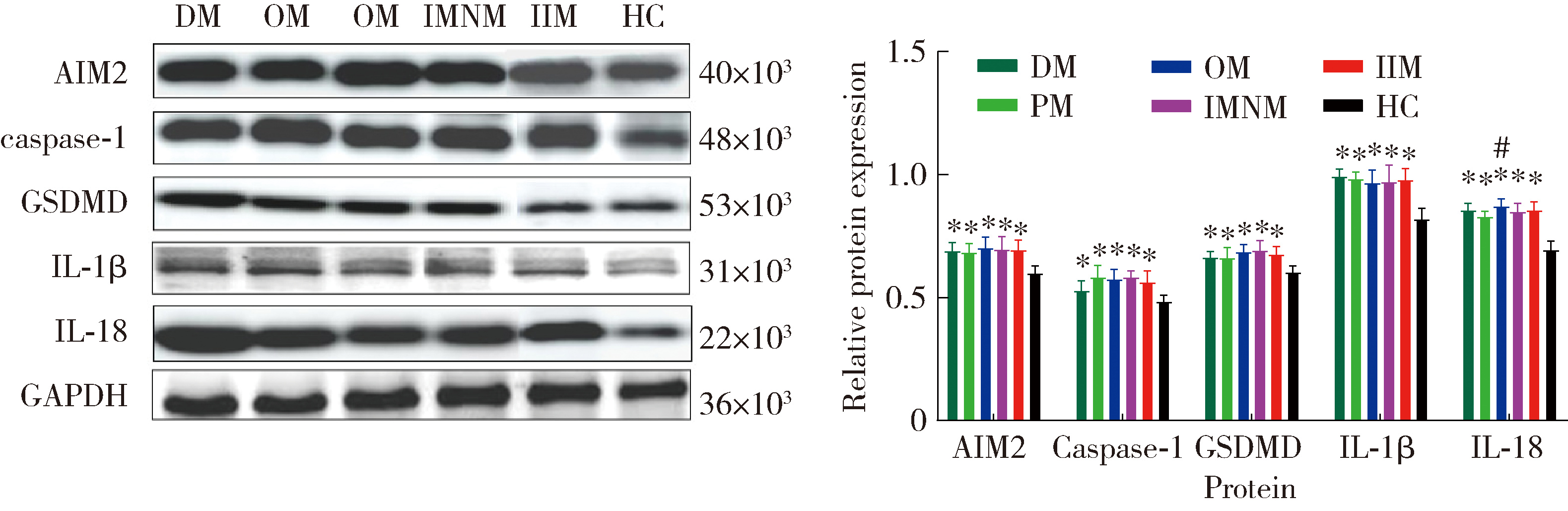
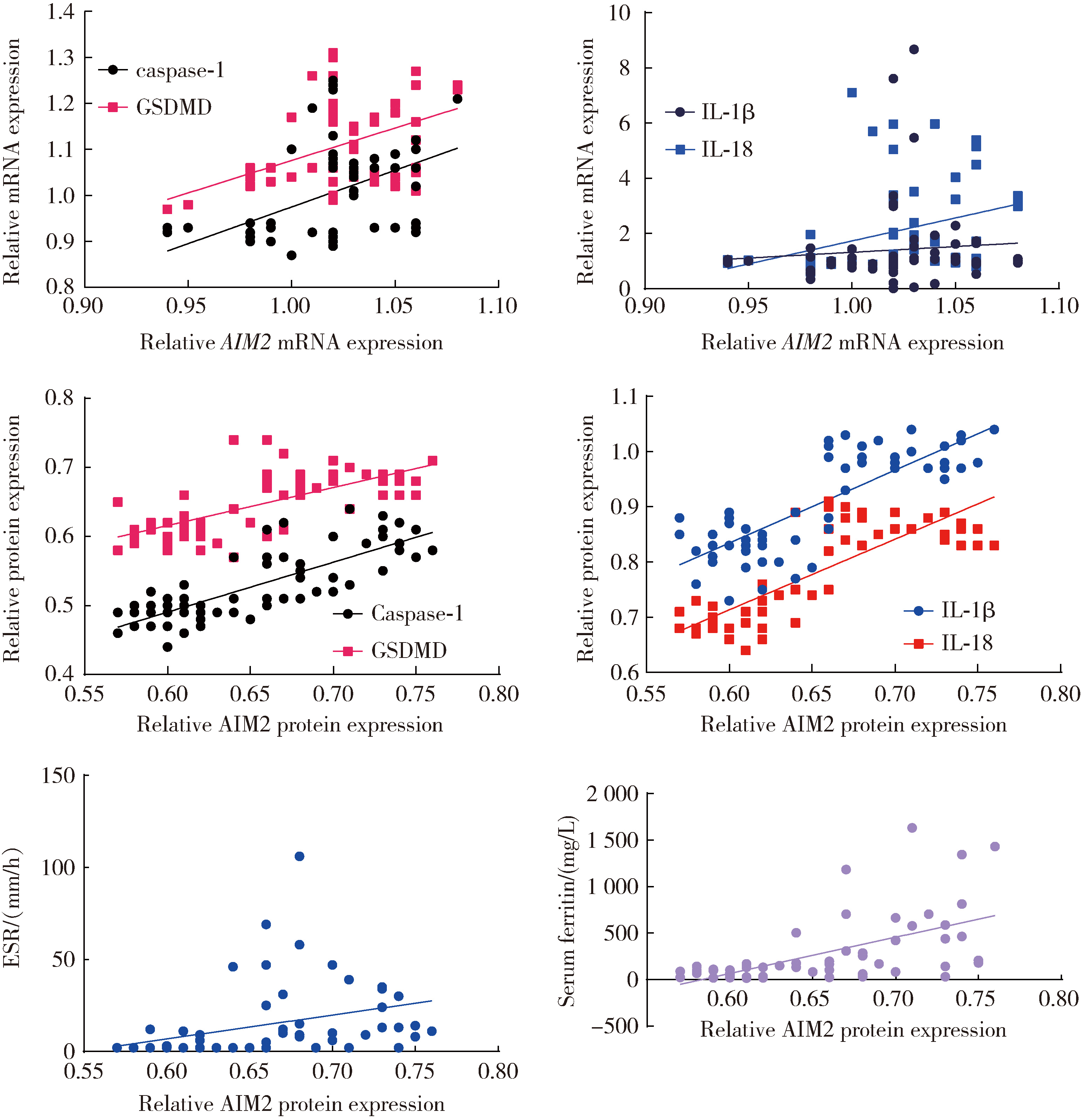
目的: 检测特发性炎性肌病(idiopathic inflammatory myopathy,IIM)患者外周血单个核细胞(peripheral blood mononuclear cell,PBMC)中黑色素瘤缺失因子2(absent in melanoma 2,AIM2)及其介导的细胞焦亡通路关键组分——半胱氨酸天冬氨酸蛋白酶1(cysteine aspartate-specific protease-1,caspase-1)和焦孔素蛋白D(gasdermin D,GSDMD)的表达,并探讨其在IIM发病机制中的作用。方法: 招募2020年5月至2022年6月于中国人民解放军北部战区总医院风湿免疫科就诊的30例IIM患者(IIM组),同期于医院体检中心招募30名性别和年龄与IIM患者相匹配的健康志愿者(健康对照组),收集研究对象临床信息、血液生化和免疫标志物,以及静脉血样本。通过荧光定量法检测血清双链DNA(double-stranded DNA,dsDNA)水平,通过实时荧光定量逆转录PCR(reverse transcription quantitative real-time PCR,RT-qPCR)检测PBMC中AIM2、caspase-1、GSDMD、白细胞介素1β(interleukin 1β,IL-1β)和IL-18的mRNA表达水平,应用蛋白免疫印迹法检测PBMC中AIM2、caspase-1、GSDMD、IL-1β和IL-18的蛋白表达水平,应用酶联免疫吸附(enzyme-linked immuno-sorbent assay,ELISA)法检测血清中IL-1β和IL-18的表达水平。结果: IIM组包含皮肌炎(dermatomyositis,DM,n=10)、多发性肌炎(polymyositis,PM,n=5)、重叠性肌炎(overlap syndrome,OM,n=11)和免疫介导的坏死性肌病(immune-mediated necrotizing myopathy,IMNM,n=4)4个亚组。与健康对照组相比,IIM组及其各亚组的血清中dsDNA、IL-1β和IL-18水平均显著增加(P<0.05)。除IMNM亚组PBMC中AIM2 mRNA与健康对照组差异无统计学意义外,IIM组及其他亚组AIM2、caspase-1和GSDMD mRNA表达均显著增加(P<0.05);除IMNM和OM亚组PBMC中IL-1β mRNA与健康对照组比较差异无统计学意义外,IIM组及其他亚组IL-1β和IL-18 mRNA表达均显著增加(P<0.05);亚组间比较表明,DM亚组PBMC中IL-1β mRNA表达明显高于OM和IMNM亚组,PM亚组IL-18 mRNA表达明显高于DM和OM亚组(P<0.05)。IIM及其各亚组的PBMC中,AIM2、caspase-1、GSDMD、IL-1β和IL-18蛋白的表达水平均显著高于健康对照组(P<0.05);各亚组间比较发现,OM亚组的IL-18蛋白表达显著高于PM亚组(P<0.05)。相关性分析表明,IIM组caspase-1、GSDMD和IL-18 mRNA与AIM2 mRNA呈正相关,caspase-1、GSDMD、IL-1β和IL-18蛋白与AIM2蛋白表达也呈正相关。结论: AIM2炎性小体介导的细胞焦亡通路可能参与IIM的发病机制,这一结论可以为研究IIM的病因及开发新的治疗方法提供理论基础。
中图分类号:
- R593.26
| 1 |
doi: 10.1136/annrheumdis-2013-205127 |
| 2 |
doi: 10.1111/j.1756-185X.2011.01669.x |
| 3 |
doi: 10.1016/S1474-4422(18)30254-0 |
| 4 |
doi: 10.1002/art.40320 |
| 5 |
doi: 10.1002/eji.201848070 |
| 6 |
doi: 10.1016/j.clim.2016.12.011 |
| 7 |
doi: 10.1016/j.imbio.2019.11.015 |
| 8 |
doi: 10.1016/j.jaut.2019.102381 |
| 9 |
doi: 10.1186/s13075-016-1033-y |
| 10 |
doi: 10.1016/j.nmd.2017.09.016 |
| 11 |
刘宁, 吴婵媛, 王迁, 等. 特发性炎性肌病核心评估指标[J]. 中华临床免疫和变态反应杂志, 2019, 13 (4): 318- 321.
|
| 12 |
潘蕾, 谢娟, 李嘉欣, 等. 特发性炎性肌病的评估与监测[J]. 临床内科杂志, 2023, 40 (3): 148- 151.
|
| 13 |
陈梦雅, 郑捷, 曹华. 皮肌炎皮损评分方法的临床应用[J]. 中华皮肤科杂志, 2017, 50 (1): 70- 72.
|
| 14 |
doi: 10.1111/bjd.18600 |
| 15 |
doi: 10.1093/rheumatology/keaa473 |
| 16 |
doi: 10.4103/0366-6999.180528 |
| 17 |
doi: 10.1172/jci.insight.134189 |
| 18 |
doi: 10.1002/art.41078 |
| 19 |
doi: 10.1016/j.immuni.2012.02.014 |
| 20 |
doi: 10.1038/nature07725 |
| 21 |
doi: 10.1189/jlb.3MR0516-224R |
| 22 |
doi: 10.1038/s41598-022-22754-4 |
| 23 |
doi: 10.1002/art.41639 |
| 24 |
doi: 10.1111/j.1365-2249.2006.03180.x |
| 25 |
doi: 10.1093/rheumatology/key222 |
| 26 |
doi: 10.1172/jci.insight.139558 |
| 27 |
|
| 28 |
doi: 10.1038/s41577-019-0228-2 |
| 29 |
doi: 10.1016/j.intimp.2021.107810 |
| 30 |
邓蕊, 柴克霞. 细胞焦亡非经典途径蛋白质在皮肌炎/多发性肌炎患者肌肉组织中的表达及意义[J]. 中华微生物学和免疫学杂志, 2021, 41 (10): 771- 777.
|
| [1] | 练益瑞, 刘静璇, 赵亮, 赵静, 臧思田, 李玉慧. 抗PM/Scl抗体相关风湿性疾病谱及其在特发性炎性肌病中的免疫学特征[J]. 北京大学学报(医学版), 2025, 57(6): 1018-1023. |
| [2] | 赖展鸿,李嘉辰,贠泽霖,张永刚,张昊,邢晓燕,邵苗,金月波,王乃迪,李依敏,李玉慧,栗占国. 特发性炎性肌病完全临床应答相关因素的单中心真实世界研究[J]. 北京大学学报(医学版), 2024, 56(2): 284-292. |
| [3] | 肖云抒,朱冯赟智,罗澜,邢晓燕,李玉慧,张学武,沈丹华. 88例重叠肌炎的临床及免疫学特征[J]. 北京大学学报(医学版), 2021, 53(6): 1088-1093. |
| [4] | 卢昕,张立宁. 肌活检在特发性炎性肌病诊断和临床分型中的价值[J]. 北京大学学报(医学版), 2018, 50(6): 949-951. |
|
||