北京大学学报(医学版) ›› 2019, Vol. 51 ›› Issue (6): 1150-1154. doi: 10.19723/j.issn.1671-167X.2019.06.030
早期糖尿病周围神经病变大鼠模型的建立
- 北京大学第一医院内分泌科, 北京 100034
Approach to creating early diabetic peripheral neuropathy rat model
Jiao HE,Ge-heng YUAN( ),Jun-qing ZHANG,Xiao-hui GUO
),Jun-qing ZHANG,Xiao-hui GUO
- Department of Endocrinology, Peking University First Hospital, Beijing 100034, China
摘要:
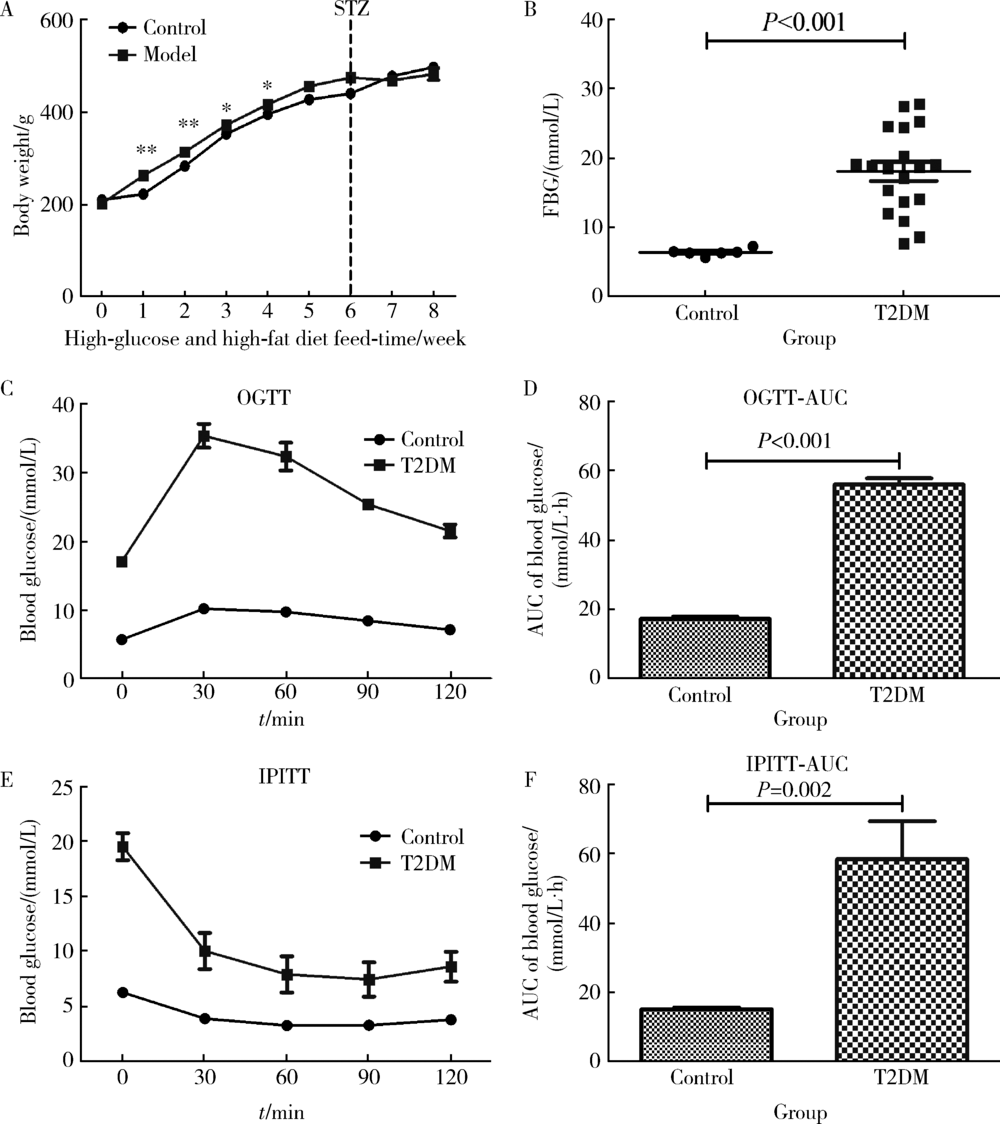
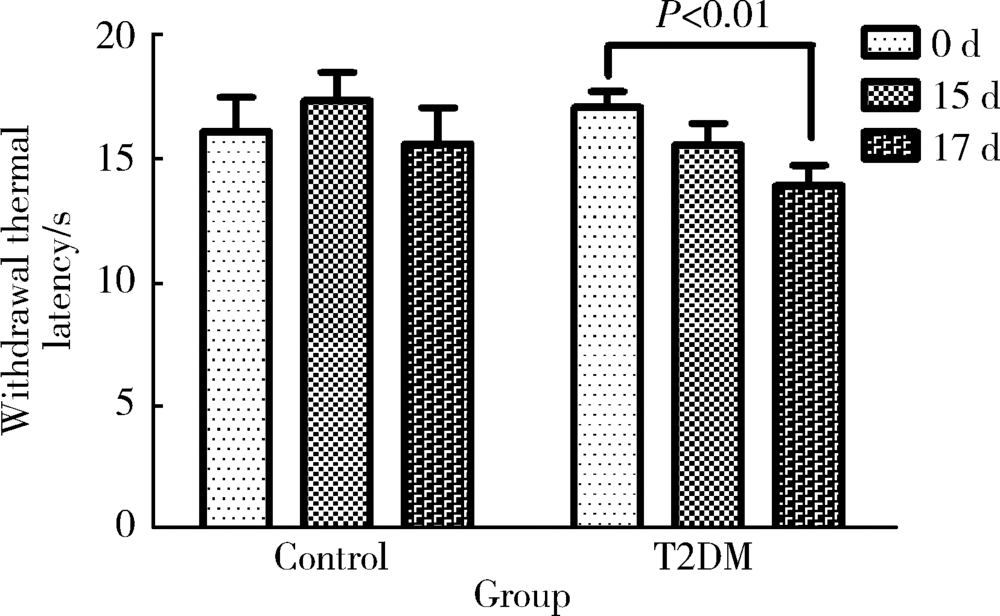
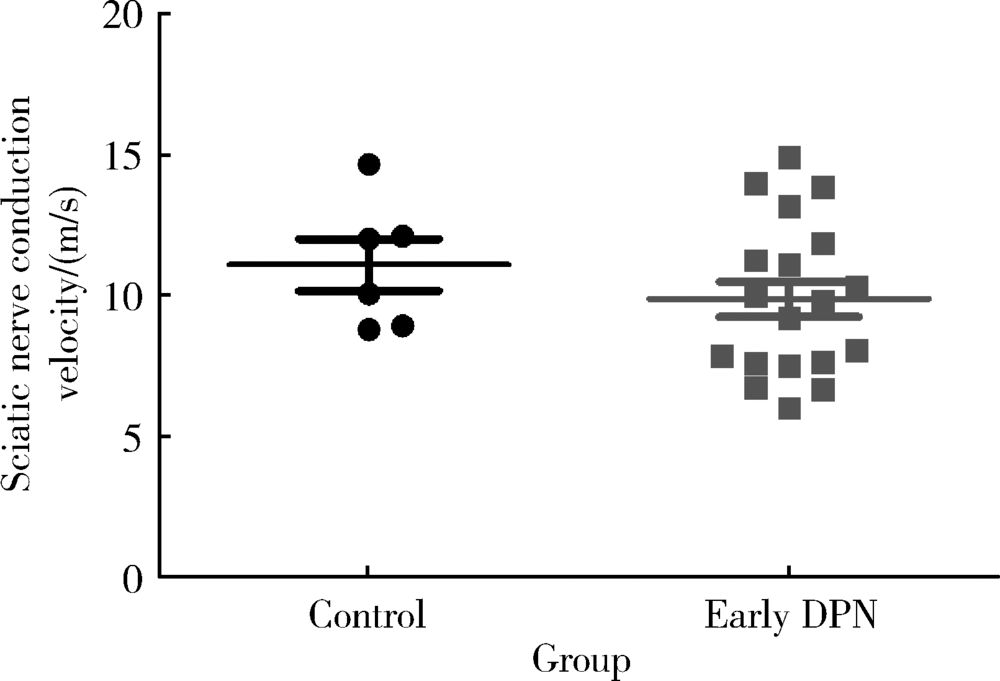
目的 旨在建立早期糖尿病周围神经病变(diabetic peripheral neuropathy, DPN)大鼠模型。方法 26只雄性SD大鼠经过适应性喂养1周后,分为对照组(n=6)和模型组(n=22)。模型组给予D12451高糖高脂饲料(碳水化合物的热量占比35%,脂肪的热量占比45%)喂养6周诱导胰岛素抵抗,对照组给予普通饲料。随后模型组按体质量给予35 mg/kg的链脲佐菌素(streptozocin,STZ)缓冲液腹腔注射诱导胰腺特异性损伤,对照组给予等量的缓冲液,48 h后测定模型组大鼠的随机血糖,大于16.7 mmol/L即认为2型糖尿病(type 2 diabetes mellitus,T2DM)造模成功。评价T2DM大鼠的一般特征,包括体质量、空腹血糖、糖耐量及胰岛素耐量。定期监测其热痛阈的变化,判断早期DPN出现的时间,最后测定大鼠的坐骨神经传导速率。结果 模型组大鼠经过高糖高脂饮食喂养6周联合STZ腹腔注射后,T2DM造模成功。T2DM组与对照组相比,空腹血糖明显升高(P<0.001),糖耐量及胰岛素耐量均明显受损(分别为P<0.001,P=0.002)。模型建立成功后第17天,T2DM组与对照组相比表现出明显的热痛觉过敏(P=0.004),两组的坐骨神经传导速率差异无统计学意义(P=0.196)。结论 高糖高脂饮食喂养大鼠6周联合35 mg/kg的STZ腹腔注射可成功诱导T2DM模型,表现为一定程度的胰岛素抵抗与胰岛素缺乏,此模型在第17天左右出现早期DPN,其中小纤维的损害早于大纤维。
中图分类号:
- R745
| [1] | Singh R, Kishore L, Kaur N . Diabetic peripheral neuropathy: current perspective and future directions[J]. Pharmacol Res, 2014,80:21-35. |
| [2] | Shi X, Chen Y, Nadeem L , et al. Beneficial effect of TNF-α inhibition on diabetic peripheral neuropathy[J]. J Neuroinflammation, 2013,10:69. |
| [3] | Bai J, Zhu Y, Dong Y . Response of gut microbiota and inflammatory status to bitter melon (Momordica charantia L.) in high fat diet induced obese rats[J]. J Ethnopharmacol, 2016,194:717-726. |
| [4] | Ishibashi K, Hara A, Fujitani Y , et al. Beneficial effects of vildagliptin combined with miglitol on glucose tolerance and islet morphology in diet-controlled db/db mice[J]. Biochem Biophys Res Commun, 2013,440(4):570-575. |
| [5] | Jolivalt CG, Frizzi KE, Guernsey L , et al. Peripheral neuropathy in mouse models of diabetes[J]. Curr Protoc Mouse Biol, 2016,6(3):223-255. |
| [6] | Balter RE, Dykstra LA . Thermal sensitivity as a measure of spontaneous morphine withdrawal in mice[J]. J Pharmacol Toxicol Methods, 2013,67(3):162-168. |
| [7] | Shi W, Ding Y, Yu A , et al. BDNF/TRK/KCC2 pathway in nicotine withdrawal-induced hyperalgesia[J]. Transl Neurosci, 2015,6(1):208-213. |
| [8] | 宋庆芳 . 游离脂肪酸与2型糖尿病周围神经病变的关系及其机制探讨[D]. 石家庄: 河北医科大学, 2010. |
| [9] | 吴庆秋 . 枸杞多糖对氧化应激诱导2型糖尿病大鼠周围神经细胞凋亡的保护作用及其机制研究[D]. 银川: 宁夏医科大学, 2010. |
| [10] | Ding Y, Dai X, Jiang Y , et al. Functional and morphological effects of grape seed proanthocyanidins on peripheral neuropathy in rats with type 2 diabetes mellitus[J]. Phytother Res, 2014,28(7):1082-1087. |
| [11] | Reed MJ, Meszaros K, Entes LJ , et al. A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat[J]. Metabolism, 2000,49(11):1390-1394. |
| [12] | Zhou JY, Zhou SW . Protection of trigonelline on experimental diabetic peripheral neuropathy[J]. Evid Based Complement Alternat Med, 2012,164219. doi: 10.1155/2012/164219. |
| [13] | 邢国平 . 糖尿病患者周围神经功能的神经电生理比较研究[D]. 天津: 天津医科大学, 2009. |
| [14] | Chéliout-Héraut F, Zrek N, Khemliche H , et al. Exploration of small fibers for testing diabetic neuropathies[J]. Joint Bone Spine, 2005,72(5):412-415. |
| [15] | Krämer HH, Rolke R, Bickel A , et al. Thermal thresholds predict painfulness of diabetic neuropathies[J]. Diabetes Care, 2004,27(10):2386-2391. |
| [16] | Hao GM, Liu YG, Wu Y , et al. The protective effect of the active components of ERPC on diabetic peripheral neuropathy in rats[J]. J Ethnopharmacol, 2017,202:162-171. |
| [17] | Xu X, Yang X, Zhang P , et al. Effects of exogenous galanin on neuropathic pain state and change of galanin and its receptors in DRG and SDH after sciatic nerve-pinch injury in rat[J]. PLoS One, 2012,7(5):e37621. |
| [18] | Liao C, Yang M, Zhong W , et al. Association of myelinated primary afferents impairment with mechanical allodynia in diabetic peripheral neuropathy: an experimental study in rats[J]. Oncotarget, 2017,8(38):64157-64169. |
| [1] | 李岩,王辉,邓莹,姚瑶,李民. 静脉输注右美托咪定对臂丛阻滞效果的随机对照研究[J]. 北京大学学报(医学版), 2018, 50(5): 845-849. |
| [2] | 邓莹,姜天乐,杨晓霞,李民,王军,郭向阳. 连续股神经阻滞联合关节周围浸润镇痛对全膝关节置换术后关节早期功能恢复的影响[J]. 北京大学学报(医学版), 2017, 49(1): 137-141. |
| [3] | 伊军,许莉,林惠华. 不同背景量连续胫神经阻滞用于跟骨手术术后镇痛的临床效果[J]. 北京大学学报(医学版), 2016, 48(2): 283-286. |
| [4] | 朱丰, 胡焱, 张伟. 连续股神经阻滞置管长度对股神经、股外侧皮神经和闭孔神经阻滞效果的影响[J]. 北京大学学报(医学版), 2013, 45(1): 145-148. |
| [5] | 伊军, 许莉, 林惠华. 连续坐骨神经阻滞镇痛时B超联合刺激导管放置技术与罗哌卡因应用浓度[J]. 北京大学学报(医学版), 2010, 42(5): 535-538. |
| [6] | 万有, , 韩济生, John E. Pintar. 孤啡肽基因敲除小鼠电针镇痛作用增强[J]. 北京大学学报(医学版), 2009, 41(3): 376-379. |
|
||