北京大学学报(医学版) ›› 2020, Vol. 52 ›› Issue (6): 1056-1062. doi: 10.19723/j.issn.1671-167X.2020.06.011
戈利木单抗治疗白塞综合征心脏大血管受累的临床分析
孙鹿希1,刘金晶1,侯云霞2,李超然1,李璐1,田新平1,曾小峰1,郑文洁1,△( )
)
- 1. 中国医学科学院 北京协和医学院 北京协和医院风湿免疫科 风湿免疫病学教育部重点实验室 协和转化医学中心 国家皮肤与免疫疾病临床医学研究中心,北京 100730
2. 内蒙古医科大学附属医院风湿免疫科,呼和浩特 010050
Clinical analysis of golimumab in the treatment of severe/refractory cardiovascular involvement in Behcet syndrome
Lu-xi SUN1,Jin-jing LIU1,Yun-xia HOU2,Chao-ran LI1,Lu LI1,Xin-ping TIAN1,Xiao-feng ZENG1,Wen-jie ZHENG1,△( )
)
- 1. Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Peking Union Medical College & Chinese Academy of Medical Sciences, Key Laboratory of Rheumatology & Clinical Immunology, Ministry of Education, National Clinical Research Center for Dermatologic and Immunologic Diseases, Beijing 100730, China
2. Department of Rheumatology, the Affiliated Hospital of Inner Mongolia Medical University, Hohhot 010050, China
摘要:
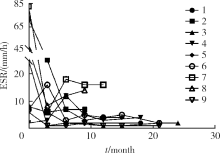
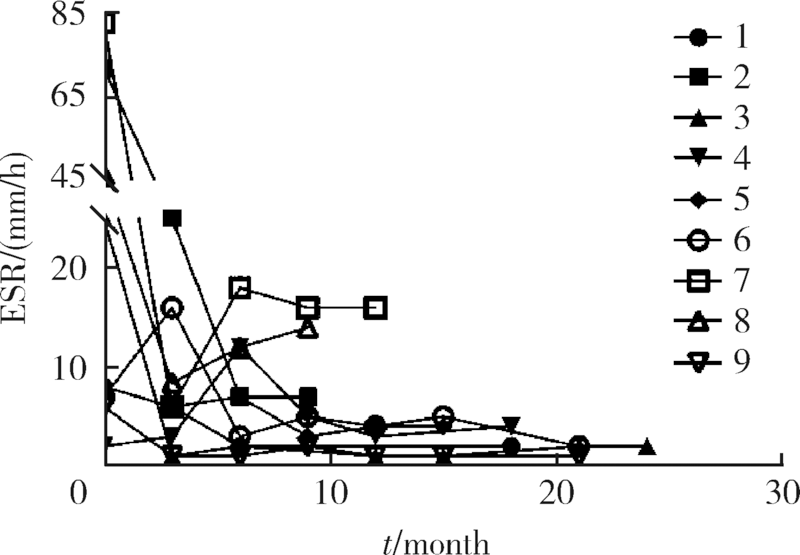
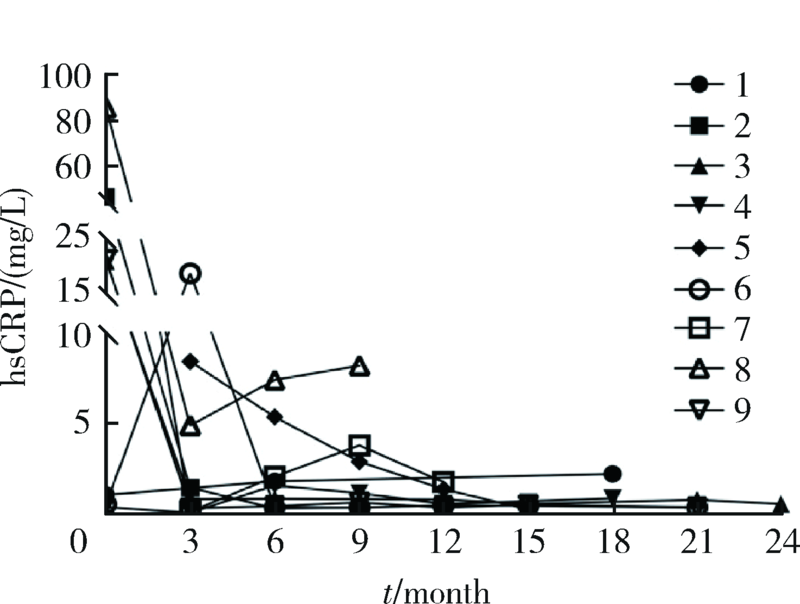
目的:探索戈利木单抗(golimumab, GOL)治疗重症/难治性心脏大血管白塞综合征(Behcet syndrome, BS)的疗效和安全性。方法:分析北京协和医院2018年2月—2020年7月使用GOL治疗的9例重症/难治性BS大血管和心脏瓣膜受累患者的临床资料,记录治疗前后红细胞沉降率 (erythrocyte sedimentation rate,ESR)、超敏C反应蛋白(high-sensitivity C-reactive protein,hsCRP)水平、临床症状和影像学变化、合并用药的种类和剂量。结果:9例患者中男性8例、女性1例,平均年龄(37.0±8.6)岁,中位病程120(60,132)个月。患者中重度主动脉瓣关闭不全7例,多发动脉瘤、静脉血栓2例。7例患者既往应用激素联合免疫抑制剂/调节剂治疗,仍出现大血管病变进展或围手术期炎症指标明显升高;5例患者病情进展,需短期内手术治疗;3例患者新近完成心脏手术但既往反复出现严重术后并发症或术前炎症未控制;1例应用托珠单抗,新发消化道溃疡,需调整用药。患者应用GOL(50 mg,每4周皮下注射一次)与糖皮质激素和免疫抑制剂/调节剂联合治疗,随访(16.3±5.6)个月,临床症状均有改善,无新发大血管病变。8例患者行手术治疗,均未出现术后并发症。治疗后炎症指标下降,ESR由16.5(6.8,52.5)mm/h降至4(2,7)mm/h,hsCRP由21.24(0.93,32.51)mg/L降至0.58(0.37,1.79)mg/L(P<0.05)。泼尼松剂量由35(15,60)mg/d减至10.0(10.0,12.5)mg/d,环磷酰胺剂量均减量,6例患者GOL停用或减量,所有患者均无明显不良反应。结论:对重症/难治性心脏大血管受累的BS患者,应用GOL与糖皮质激素和免疫抑制剂/调节剂联合治疗,有助于安全、有效控制病情,降低炎症反应,减少术后严重并发症和糖皮质激素用量。
中图分类号:
- R597.9
| [1] |
Takeuchi M, Kastner DL, Remmers EF. The immunogenetics of Behcet’s disease: A comprehensive review[J]. J Autoimmun, 2015,64:137-148.
pmid: 26347074 |
| [2] |
Zeidan MJ, Saadoun D, Garrido M, et al. Behcet’s disease physiopathology: A contemporary review[J]. Auto Immun Highlights, 2016,7(1):4.
doi: 10.1007/s13317-016-0074-1 pmid: 26868128 |
| [3] |
Sakane T, Takeno M, Suzuki N, et al. Behcet’s disease[J]. N Engl J Med, 1999,341(17):1284-1291.
doi: 10.1056/NEJM199910213411707 pmid: 10528040 |
| [4] |
Geri G, Wechsler B, Thi Huong du L, et al. Spectrum of cardiac lesions in Behcet’s disease: A series of 52 patients and review of the literature[J]. Medicine (Baltimore), 2012,91(1):25-34.
doi: 10.1097/MD.0b013e3182428f49 |
| [5] | 郑文洁. 重视白塞病血管病变[J]. 中华风湿病学杂志, 2016,20(12):793-795. |
| [6] |
Merashli M, Eid RE, Uthman I. A review of current management of vasculo-Behcet’s[J]. Curr Opin Rheumatol, 2018,30(1):50-56.
doi: 10.1097/BOR.0000000000000458 pmid: 29076891 |
| [7] |
Vitale A, Emmi G, Lopalco G, et al. Long-term efficacy and safety of golimumab in the treatment of multirefractory Behcet’s disease[J]. Clin Rheumatol, 2017,36(9):2063-2069.
pmid: 28401434 |
| [8] |
Fabiani C, Sota J, Rigante D, et al. Rapid and sustained efficacy of golimumab in the treatment of multirefractory uveitis associated with Behcet’s disease[J]. Ocul Immunol Inflamm, 2019,27(1):58-63.
doi: 10.1080/09273948.2017.1351573 pmid: 28981395 |
| [9] |
International Team for the Revision of the International Criteria for Behcet’s Disease (ITR-ICBD). The International Criteria for Behcet’s Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria[J]. J Eur Acad Dermatol Venereol, 2014,28(3):338-347.
pmid: 23441863 |
| [10] |
Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group[J]. Am J Clin Oncol, 1982,5(6):649-655.
pmid: 7165009 |
| [11] |
Duzgun N, Ates A, Aydintug OT, et al. Characteristics of vascular involvement in Behcet’s disease[J]. Scand J Rheumatol, 2006,35(1):65-68.
pmid: 16467046 |
| [12] |
Seyahi E, Yazici H. Behcet’s syndrome: Pulmonary vascular disease[J]. Curr Opin Rheumatol, 2015,27(1):18-23.
doi: 10.1097/BOR.0000000000000131 pmid: 25415527 |
| [13] | Alibaz-Oner F, Karadeniz A, Ylmaz S, et al. Behcet disease with vascular involvement: effects of different therapeutic regimens on the incidence of new relapses[J]. Medicine (Baltimore), 2015,94(6):e494. |
| [14] |
Hamuryudan V, Er T, Seyahi E, et al. Pulmonary artery aneurysms in Behcet syndrome[J]. Am J Med, 2004,117(11):867-870.
doi: 10.1016/j.amjmed.2004.05.027 pmid: 15589493 |
| [15] | Saadoun D, Asli B, Wechsler B, et al. Long-term outcome of arterial lesions in Behcet disease: a series of 101 patients[J]. Medicine (Baltimore), 2012,91(1):18-24. |
| [16] | Seyahi E, Melikoglu M, Akman C, et al. Pulmonary artery involvement and associated lung disease in Behcet disease: a series of 47 patients[J]. Medicine (Baltimore), 2012,91(1):35-48. |
| [17] |
Emmi G, Silvestri E, Squatrito D, et al. Thrombosis in vasculitis: from pathogenesis to treatment[J]. Thromb J, 2015,13:15.
doi: 10.1186/s12959-015-0047-z pmid: 25883536 |
| [18] |
Onur E, Kabaroglu C, Inanir I, et al. Oxidative stress impairs endothelial nitric oxide levels in Behcet’s disease[J]. Cutan Ocul Toxicol, 2011,30(3):217-220.
doi: 10.3109/15569527.2011.554480 pmid: 21345164 |
| [19] |
Pfeiler S, Stark K, Massberg S, et al. Propagation of thrombosis by neutrophils and extracellular nucleosome networks[J]. Haematologica, 2017,102(2):206-213.
doi: 10.3324/haematol.2016.142471 pmid: 27927771 |
| [20] |
Zhou ZY, Chen SL, Shen N, et al. Cytokines and Behcet’s disease[J]. Autoimmun Rev, 2012,11(10):699-704.
pmid: 22197901 |
| [21] |
Tong B, Liu X, Xiao J, et al. Immunopathogenesis of Behcet’s disease[J]. Front Immunol, 2019,10:665.
doi: 10.3389/fimmu.2019.00665 pmid: 30984205 |
| [22] | van der Houwen T, van Laar J. Behet’s disease, and the role of TNF-alpha and TNF-alpha blockers[J]. Int J Mol Sci, 2020,21(9):3072. |
| [23] |
Lee EB, Kim JY, Lee YJ, et al. TNF and TNF receptor polymorphisms in Korean Behcet’s disease patients[J]. Hum Immunol, 2003,64(6):614-620.
doi: 10.1016/s0198-8859(03)00057-0 pmid: 12770792 |
| [24] |
Dalghous AM, Freysdottir J, Fortune F. Expression of cytokines, chemokines, and chemokine receptors in oral ulcers of patients with Behcet’s disease (BD) and recurrent aphthous stomatitis is Th1-associated, although Th2-association is also observed in patients with BD[J]. Scand J Rheumatol, 2006,35(6):472-475.
doi: 10.1080/03009740600905380 pmid: 17343257 |
| [25] |
El-Asrar AM, Struyf S, Kangave D, et al. Cytokine profiles in aqueous humor of patients with different clinical entities of endogenous uveitis[J]. Clin Immunol, 2011,139(2):177-184.
doi: 10.1016/j.clim.2011.01.014 pmid: 21334264 |
| [26] |
Emmi G, Silvestri E, Bella CD, et al. Cytotoxic Th1 and Th17 cells infiltrate the intestinal mucosa of Behcet patients and exhibit high levels of TNF-alpha in early phases of the disease[J]. Medicine (Baltimore), 2016,95(49):e5516.
doi: 10.1097/MD.0000000000005516 |
| [27] |
Aksoy A, Yazici A, Omma A, et al. Efficacy of TNFalpha inhibitors for refractory vascular Behcet’s disease: A multicenter observational study of 27 patients and a review of the literature[J]. Int J Rheum Dis, 2020,23(2):256-261.
doi: 10.1111/1756-185X.13778 pmid: 31976619 |
| [28] |
Chan E, Sangle SR, Coghlan JG, et al. Pulmonary artery aneurysms in Behcet’s disease treated with anti-TNFalpha: A case series and review of the literature[J]. Autoimmun Rev, 2016,15(4):375-378.
doi: 10.1016/j.autrev.2016.01.003 pmid: 26777307 |
| [29] |
Hamuryudan V, Seyahi E, Ugurlu S, et al. Pulmonary artery involvement in Behcet’s syndrome: Effects of anti-TNF treatment[J]. Semin Arthritis Rheum, 2015,45(3):369-373.
doi: 10.1016/j.semarthrit.2015.06.008 pmid: 26190564 |
| [30] |
Desbois AC, Biard L, Addimanda O, et al. Efficacy of anti-TNF alpha in severe and refractory major vessel involvement of Behcet’s disease: A multicenter observational study of 18 patients[J]. Clin Immunol, 2018,197:54-59.
doi: 10.1016/j.clim.2018.08.004 pmid: 30125675 |
| [31] |
Hatemi G, Christensen R, Bang D, et al. 2018 update of the EULAR recommendations for the management of Behcet’s syndrome[J]. Ann Rheum Dis, 2018,77(6):808-818.
doi: 10.1136/annrheumdis-2018-213225 pmid: 29625968 |
| [32] | 李璐, 刘金晶, 郁欣, 等. 抗肿瘤坏死因子α单抗治疗16例重症/难治性血管白塞病的疗效与安全性[J]. 中华内科杂志, 2020,59(4):303-308. |
| [33] | Thomas SS, Borazan N, Barroso N, et al. Comparative immunogenicity of TNF inhibitors: impact on clinical efficacy and tolerability in the management of autoimmune diseases. A systematic review and meta-analysis[J]. Bio Drugs, 2015,29(4):241-258. |
| [34] |
Svedbom A, Storck C, Kachroo S, et al. Persistence with golimumab in immune-mediated rheumatic diseases: a systematic review of real-world evidence in rheumatoid arthritis, axial spondyloarthritis, and psoriatic arthritis[J]. Patient Prefer Adherence, 2017,11:719-729.
doi: 10.2147/PPA.S128665 pmid: 28435230 |
| [35] |
Kawalec P, Pilc A. An indirect comparison of infliximab versus adalimumab or golimumab for active ulcerative colitis[J]. Arch Med Sci, 2016,12(5):1097-1109.
pmid: 27695502 |
| [36] |
Shealy D, Cai A, Staquet K, et al. Characterization of golimu-mab, a human monoclonal antibody specific for human tumor necrosis factor α[J]. MAbs, 2010,2(4):428-439.
pmid: 20519961 |
| [37] |
Sánchez-Cano D, Callejas-Rubio JL, Ruiz-Villaverde R, et al. Off-label uses of anti-TNF therapy in three frequent disorders: Behcet’s disease, sarcoidosis, and noninfectious uveitis[J]. Mediators Inflamm, 2013,2013:286857. doi: 10.1155/2013/286857.
doi: 10.1155/2013/286857 pmid: 23983404 |
| [38] |
Zhou H, Jang H, Fleischmann RM, et al. Pharmacokinetics and safety of golimumab, a fully human anti-TNF-alpha monoclonal antibody, in subjects with rheumatoid arthritis[J]. J Clin Pharmacol, 2007,47(3):383-396.
doi: 10.1177/0091270006298188 pmid: 17322150 |
| [39] |
Keystone EC, Genovese MC, Klareskog L, et al. Golimumab, a human antibody to tumour necrosis factor alpha given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD Study[J]. Ann Rheum Dis, 2009,68(6):789-796.
doi: 10.1136/ard.2008.099010 pmid: 19066176 |
| [40] |
Smolen JS, Kay J, Doyle MK, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, rando-mised, double-blind, placebo-controlled, phase III trial[J]. Lancet, 2009,374(9685):210-221.
doi: 10.1016/S0140-6736(09)60506-7 |
| [41] |
Boyce EG, Halilovic J, Stan-Ugbene O. Golimumab: Review of the efficacy and tolerability of a recently approved tumor necrosis factor-alpha inhibitor[J]. Clin Ther, 2010,32(10):1681-1703.
doi: 10.1016/j.clinthera.2010.09.003 |
| [42] |
Pelechas E, Voulgari PV, Drosos AA. Golimumab for rheumatoid arthritis[J]. J Clin Med, 2019,8(3):387.
doi: 10.3390/jcm8030387 |
| [43] |
Papagoras C, Voulgari PV, Drosos AA. Golimumab, the newest TNF-alpha blocker, comes of age[J]. Clin Exp Rheumatol, 2015,33(4):570-577.
pmid: 25602858 |
| [44] |
Pappas DA, St John G, Etzel CJ, et al. Comparative effectiveness of first-line tumour necrosis factor inhibitor versus non-tumour necrosis factor inhibitor biologics and targeted synthetic agents in patients with rheumatoid arthritis: results from a large US registry study [J]. Ann Rheum Dis, 2020, annrheumdis-2020-217209. doi: 10.1136/annrheumdis-2020-217209.
doi: 10.1136/annrheumdis-2020-218186 pmid: 33243781 |
| [45] |
Yokota S, Imagawa T, Mori M, et al. Efficacy and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled, withdrawal phase Ⅲ trial[J]. Lancet, 2008,371(9617):998-1006.
doi: 10.1016/S0140-6736(08)60454-7 pmid: 18358927 |
| [46] |
Strangfeld A, Richter A, Siegmund B, et al. Risk for lower intestinal perforations in patients with rheumatoid arthritis treated with tocilizumab in comparison to treatment with other biologic or conventional synthetic DMARDs[J]. Ann Rheum Dis, 2017,76(3):504-510.
doi: 10.1136/annrheumdis-2016-209773 pmid: 27405509 |
| [1] | 严冬,郑文洁. α干扰素在白塞综合征中的应用进展[J]. 北京大学学报(医学版), 2020, 52(6): 1166-1170. |
| [2] | 禹琛,李春,范洋溢,徐燕. 白塞综合征合并急性格林巴利综合征1例[J]. 北京大学学报(医学版), 2020, 52(6): 1146-1149. |
| [3] | 谢进生, 李章红, 乔志钰, 徐保卫, 许李力, 孙衍庆. 主动脉瓣病变合并升主动脉扩张的"背心式"人工血管包裹术及其应用效果[J]. 北京大学学报(医学版), 2007, 39(2): 186-188. |
|
||