北京大学学报(医学版) ›› 2021, Vol. 53 ›› Issue (6): 1026-1031. doi: 10.19723/j.issn.1671-167X.2021.06.003
类风湿关节炎患者趋化因子CXCL9和CXCL10在骨侵蚀中的作用
- 北京大学人民医院风湿免疫科,风湿病机制及免疫诊断北京市重点实验室,北京 100044
Effect of chemokines CXCL9 and CXCL10 on bone erosion in patients with rheumatoid arthritis
ZHONG Hua,XU Li-ling,BAI Ming-xin,SU Yin( )
)
- Beijing Key Laboratory for Rheumatism Mechanism and Immune Diagnosis, Beijing 100044, China
摘要:
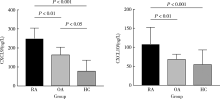
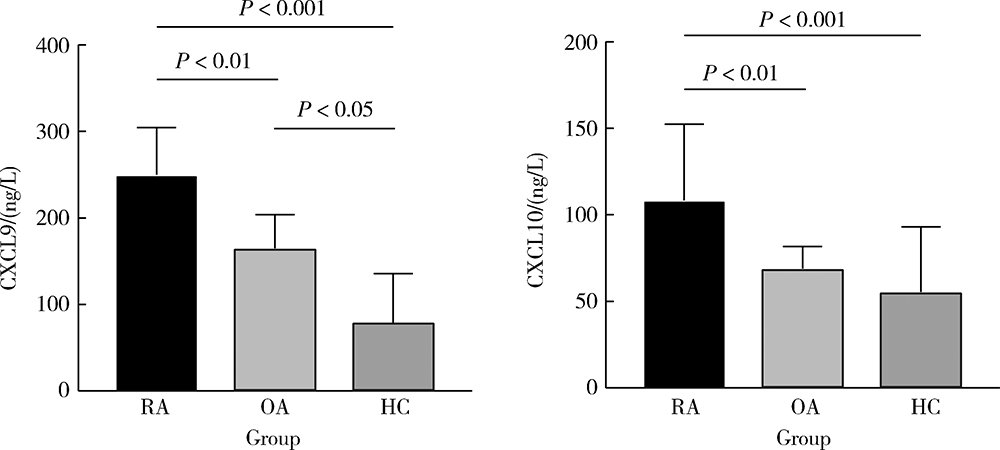
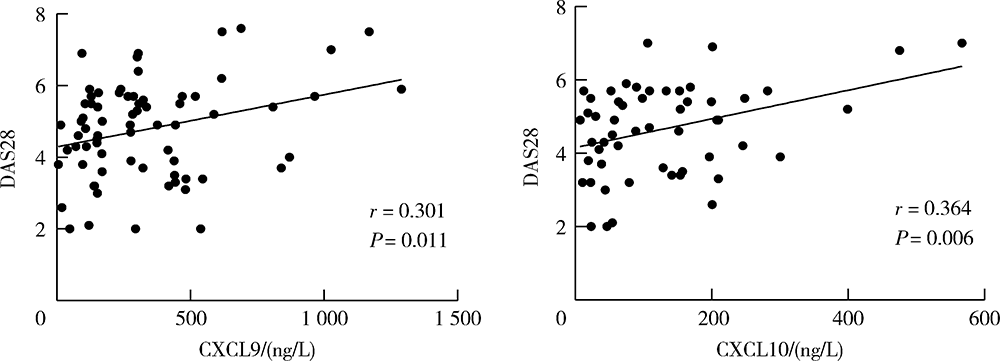
目的:检测趋化因子CXCL9和CXCL10在类风湿关节炎(rheumatoid arthritis, RA)患者外周血中的水平,分析其对RA发生骨侵蚀的作用,探讨CXCL9和CXCL10在RA中的临床意义。方法:采用酶联免疫吸附试验(enzyme linked immunosorbent assay, ELISA)检测105例RA患者、90例骨关节炎(osteoarthritis, OA)患者和25例健康对照者(healthy control, HC)血清CXCL9、CXCL10水平并比较各组间差异,分析其与RA临床特征、实验室指标、疾病活动性及骨侵蚀的相关性,采用Logistic回归分析血清CXCL9和CXCL10水平与RA患者骨侵蚀的相关性。结果:RA组患者血清CXCL9、CXCL10水平显著高于OA组和HC组(P<0.01、P<0.01),RA患者血清CXCL9水平与肿胀关节数(swollen joints, SJC)、类风湿因子(rheumatoid factor, RF)呈正相关(P<0.05),血清CXCL10水平与压痛关节数(tender joints, TJC)、SJC、C反应蛋白(C-reactive protein, CRP)、免疫球蛋白(immunoglobulin, Ig)A、IgM、RF及抗环瓜氨酸多肽抗体(anti-cyclic citrullinated peptide antibody,ACPA)呈正相关(P<0.05)。此外,血清CXCL9、CXCL10水平均与RA疾病活动度评分(disease activity score 28, DAS28)呈正相关(P=0.013、P=0.006),且高疾病活动度组(DAS28≥5.1)的血清CXCL9、CXCL10水平显著高于中低疾病活动度组(DAS28<5.1,P<0.05)。Logistic回归分析提示,病程长、高疾病活动度及血清CXCL9水平升高与RA患者发生骨侵蚀相关(P<0.05)。结论:RA患者血清趋化因子CXCL9和CXCL10的表达水平升高,与RA疾病活动性及骨侵蚀具有相关性,可能参与了RA的发病及骨破坏过程。
中图分类号:
- R593.22
| [1] |
Sparks JA. Rheumatoid arthritis [J]. Ann Intern Med, 2019, 170(1): ITC1-ITC16.
doi: 10.7326/AITC201901010 |
| [2] |
Zhu H, Li R, Da Z, et al. Remission assessment of rheumatoid arthritis in daily practice in China: A cross-sectional observational study[J]. Clin Rheumatol, 2018, 37(3):597-605.
doi: 10.1007/s10067-017-3850-z |
| [3] |
Zhou Y, Wang X, An Y, et al. Disability and health-related quality of life in Chinese patients with rheumatoid arthritis: A cross-sectional study[J]. Int J Rheum Dis, 2018, 21(9):1709-1715.
doi: 10.1111/apl.2018.21.issue-9 |
| [4] |
Poeta VM, Massara M, Capucetti A, et al. Chemokines and chemokine receptors: new targets for cancer immunotherapy[J]. Front Immunol, 2019, 10:379.
doi: 10.3389/fimmu.2019.00379 |
| [5] |
Susek KH, Karvouni M, Alici E, et al. The role of CXC chemokine receptors 1-4 on immune cells in the tumor microenvironment[J]. Front Immunol, 2018, 9:2159.
doi: 10.3389/fimmu.2018.02159 |
| [6] |
Tokunaga R, Zhang W, Naseem M, et al. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation: A target for novel cancer therapy[J]. Cancer Treat Rev, 2018, 63:40-47.
doi: S0305-7372(17)30199-8 pmid: 29207310 |
| [7] |
McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis[J]. N Engl J Med, 2011, 365(23):2205-2219.
doi: 10.1056/NEJMra1004965 |
| [8] |
Muntyanu A, Abji F, Liang K, et al. Differential gene and protein expression of chemokines and cytokines in synovial fluid of patients with arthritis[J]. Arthritis Res Ther, 2016, 18(1):296.
doi: 10.1186/s13075-016-1196-6 |
| [9] |
Antonelli A, Ferrari SM, Giuggioli D, et al. Chemokine (C-X-C motif) ligand CXCL10 in autoimmune diseases[J]. Autoimmun Rev, 2014, 13(3):272-280.
doi: 10.1016/j.autrev.2013.10.010 |
| [10] |
Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative[J]. Arthritis Rheum, 2010, 62(9):2569-2581.
doi: 10.1002/art.27584 |
| [11] |
Zhang W, Doherty M, Peat G, et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis[J]. Ann Rheum Dis, 2010, 69(3):483-489.
doi: 10.1136/ard.2009.113100 pmid: 19762361 |
| [12] |
Prevoo ML, van’t Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis[J]. Arthritis Rheum, 1995, 38(1):44-48.
doi: 10.1002/art.v38:1 |
| [13] |
Fransen J, van Riel PL. The disease activity score and the EULAR response criteria [J]. Rheum Dis Clin North Am, 2009, 35(4): 745-757, vii-viii.
doi: 10.1016/j.rdc.2009.10.001 |
| [14] |
Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis[J]. Arthritis Rheum, 1988, 31(3):315-324.
doi: 10.1002/(ISSN)1529-0131 |
| [15] | Ostergaard M, Peterfy C, Conaghan P, et al. OMERACT rheumatoid arthritis magnetic resonance imaging studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system[J]. J Rheumatol, 2003, 30(6):1385-1386. |
| [16] |
Bruyn GA, Hanova P, Iagnocco A, et al. Ultrasound definition of tendon damage in patients with rheumatoid arthritis. Results of a OMERACT consensus-based ultrasound score focusing on the diagnostic reliability[J]. Ann Rheum Dis, 2014, 73(11):1929-1934.
doi: 10.1136/annrheumdis-2013-203596 |
| [17] |
Zeidler H. The need to better classify and diagnose early and very early rheumatoid arthritis[J]. J Rheumatol, 2012, 39(2):212-217.
doi: 10.3899/jrheum.110967 |
| [18] |
Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: Positioning cells for host defense and immunity[J]. Annu Rev Immunol, 2014, 32:659-702.
doi: 10.1146/annurev-immunol-032713-120145 pmid: 24655300 |
| [19] |
Korniejewska A, McKnight AJ, Johnson Z, et al. Expression and agonist responsiveness of CXCR3 variants in human T lymphocytes[J]. Immunology, 2011, 132(4):503-515.
doi: 10.1111/j.1365-2567.2010.03384.x pmid: 21255008 |
| [20] |
Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses[J]. Adv Immunol, 2007, 96:41-101.
pmid: 17981204 |
| [21] |
Farber JM. Mig and IP-10: CXC chemokines that target lymphocytes[J]. J Leukoc Biol, 1997, 61(3):246-257.
doi: 10.1002/jlb.1997.61.issue-3 |
| [22] |
Kwak HB, Ha H, Kim HN, et al. Reciprocal cross-talk between RANKL and interferon-gamma-inducible protein 10 is responsible for bone-erosive experimental arthritis[J]. Arthritis Rheum, 2008, 58(5):1332-1342.
doi: 10.1002/(ISSN)1529-0131 |
| [23] |
Kraan MC, Patel DD, Haringman JJ, et al. The development of clinical signs of rheumatoid synovial inflammation is associated with increased synjournal of the chemokine CXCL8 (interleukin-8)[J]. Arthritis Res, 2001, 3(1):65-71.
pmid: 11178128 |
| [1] | 刘东武, 陈杰, 高明利, 于静. 类风湿关节炎伴发淋巴结Castleman样病理改变1例[J]. 北京大学学报(医学版), 2024, 56(5): 928-931. |
| [2] | 黄会娜,赵静,赵祥格,白自然,李霞,王冠. 乳酸对类风湿关节炎患者外周血CD4+T细胞亚群的调控作用[J]. 北京大学学报(医学版), 2024, 56(3): 519-525. |
| [3] | 汤晓菲,李永红,丁秋玲,孙卓,张阳,王育梅,田美伊,刘坚. 类风湿关节炎患者下肢深静脉血栓发病率及危险因素[J]. 北京大学学报(医学版), 2024, 56(2): 279-283. |
| [4] | 邹雪,白小娟,张丽卿. 艾拉莫德联合托法替布治疗难治性中重度类风湿关节炎的疗效[J]. 北京大学学报(医学版), 2023, 55(6): 1013-1021. |
| [5] | 吴琦,蔡月明,何娟,黄文蒂,王庆文. 血脂异常与类风湿关节炎肺间质病变的相关性分析[J]. 北京大学学报(医学版), 2023, 55(6): 982-992. |
| [6] | 张警丰,金银姬,魏慧,姚中强,赵金霞. 体重指数与类风湿关节炎临床特征的相关性分析[J]. 北京大学学报(医学版), 2023, 55(6): 993-999. |
| [7] | 金银姬,孙琳,赵金霞,刘湘源. 血清IgA型抗鼠科肉瘤病毒癌基因同源物B1抗体在类风湿关节炎中的意义[J]. 北京大学学报(医学版), 2023, 55(4): 631-635. |
| [8] | 蔡文心,李仕成,刘一鸣,梁如玉,李静,郭建萍,胡凡磊,孙晓麟,李春,刘栩,叶华,邓立宗,李茹,栗占国. 类风湿关节炎临床分层及其特征的横断面研究[J]. 北京大学学报(医学版), 2022, 54(6): 1068-1073. |
| [9] | 程昉,杨邵英,房星星,王璇,赵福涛. CCL28-CCR10通路在类风湿关节炎单核细胞迁移中的作用[J]. 北京大学学报(医学版), 2022, 54(6): 1074-1078. |
| [10] | 刘蕊,赵金霞,闫良. 类风湿关节炎合并下肢静脉血栓患者的临床特点[J]. 北京大学学报(医学版), 2022, 54(6): 1079-1085. |
| [11] | 张警丰,金银姬,魏慧,姚中强,赵金霞. 类风湿关节炎患者生活质量与疾病活动度的横断面研究[J]. 北京大学学报(医学版), 2022, 54(6): 1086-1093. |
| [12] | 高超,陈立红,王莉,姚鸿,黄晓玮,贾语博,刘田. 类风湿关节炎合并纤维肌痛简易分类标准的临床验证[J]. 北京大学学报(医学版), 2022, 54(2): 278-282. |
| [13] | 娄雪,廖莉,李兴珺,王楠,刘爽,崔若玫,徐健. 类风湿关节炎患者外周血TWEAK基因启动子区甲基化状态及其表达[J]. 北京大学学报(医学版), 2021, 53(6): 1020-1025. |
| [14] | 罗靓,霍文岗,张钦,李春. 类风湿关节炎合并角膜溃疡的临床特点和相关因素分析[J]. 北京大学学报(医学版), 2021, 53(6): 1032-1036. |
| [15] | 张璐,胡小红,陈澄,蔡月明,王庆文,赵金霞. 类风湿关节炎初治患者颈椎失稳情况及临床特征[J]. 北京大学学报(医学版), 2021, 53(6): 1049-1054. |
|
||