北京大学学报(医学版) ›› 2022, Vol. 54 ›› Issue (3): 400-411. doi: 10.19723/j.issn.1671-167X.2022.03.003
姜黄素干预通过促进自噬改善氯化锰所致的大鼠神经行为损伤
- 北京大学公共卫生学院毒理学系,食品安全毒理学研究与评价北京市重点实验室,北京 100191
Curcumin alleviates the manganese-induced neurotoxicity by promoting autophagy in rat models of manganism
Li-ye LAI,Chang-song DOU,Cui-na ZHI,Jie CHEN,Xue MA,Peng ZHAO,Bi-yun YAO*( )
)
- Department of Toxicology, Peking University School of Public Health, Beijing Key Laboratory of Toxicological Research and Risk Assessment for Food Safety, Beijing 100191, China
摘要:
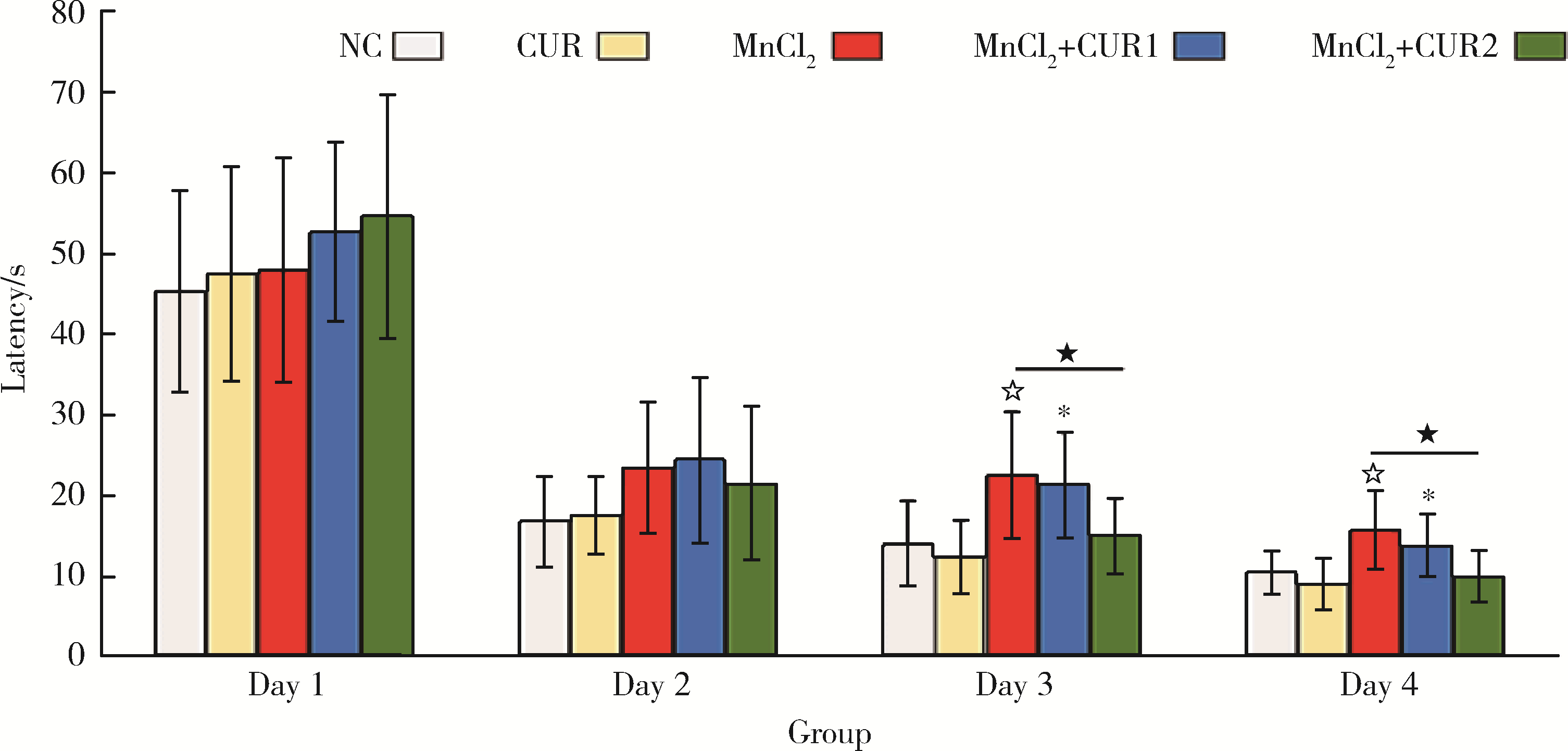
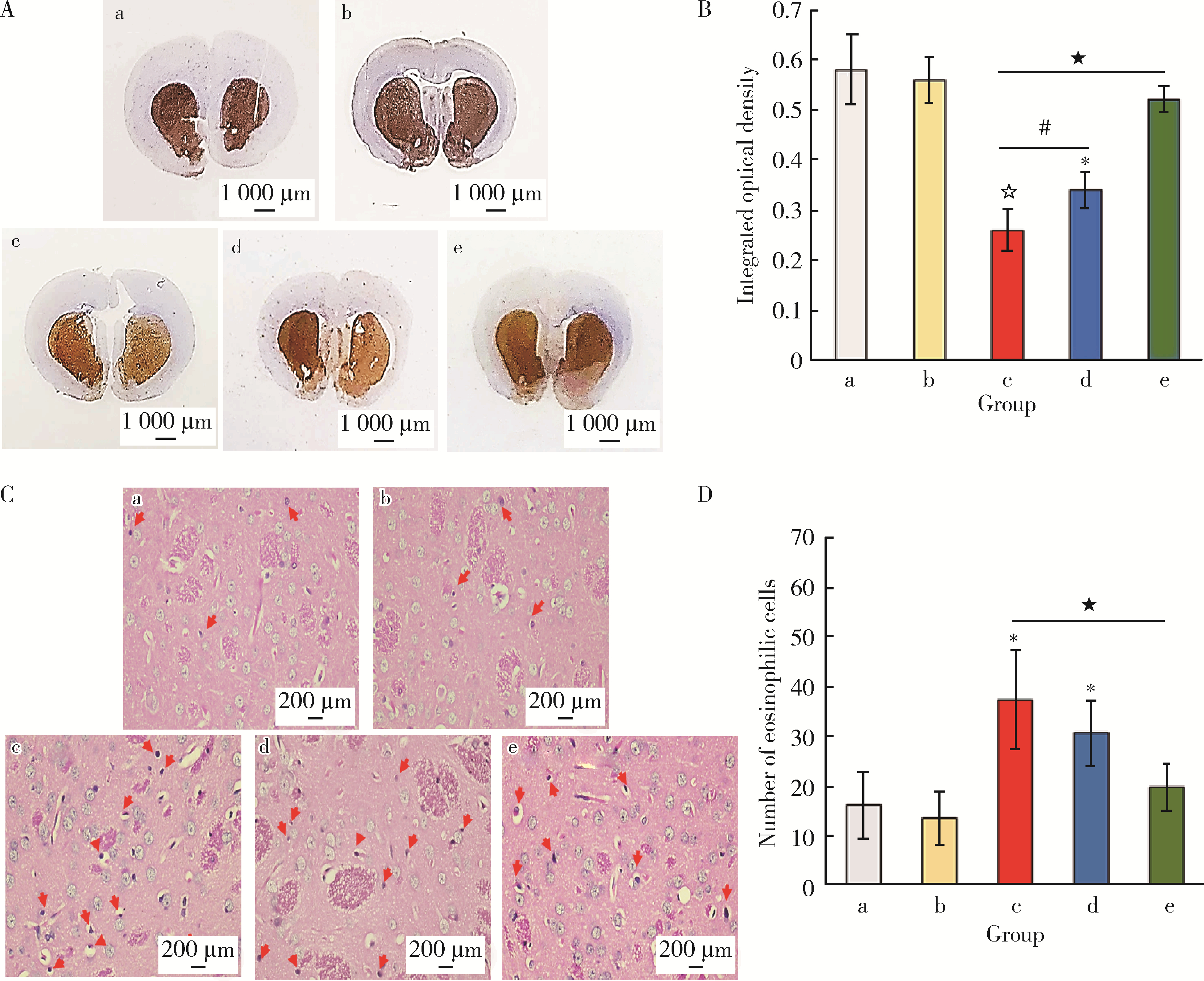
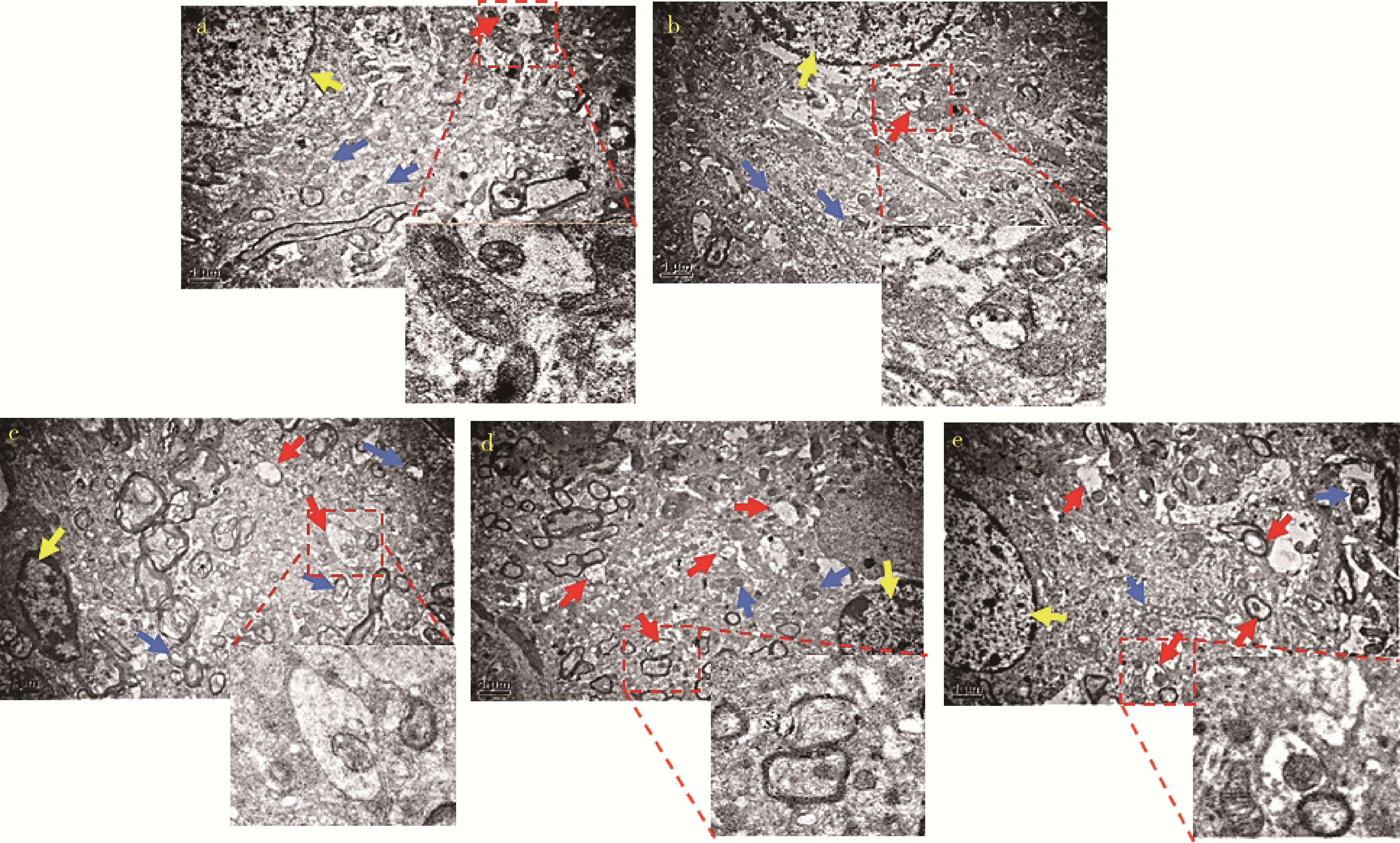
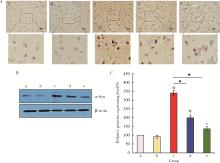
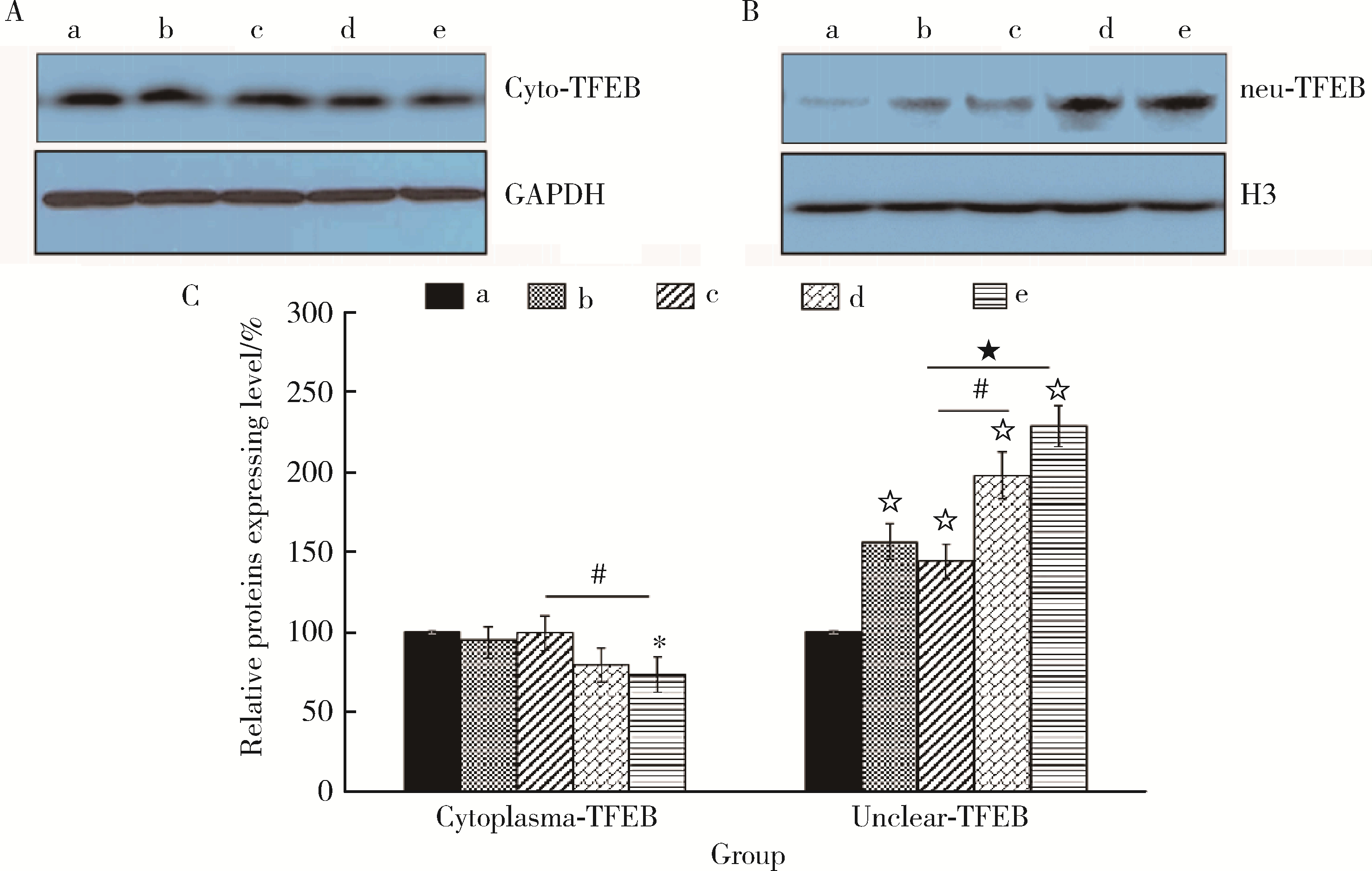
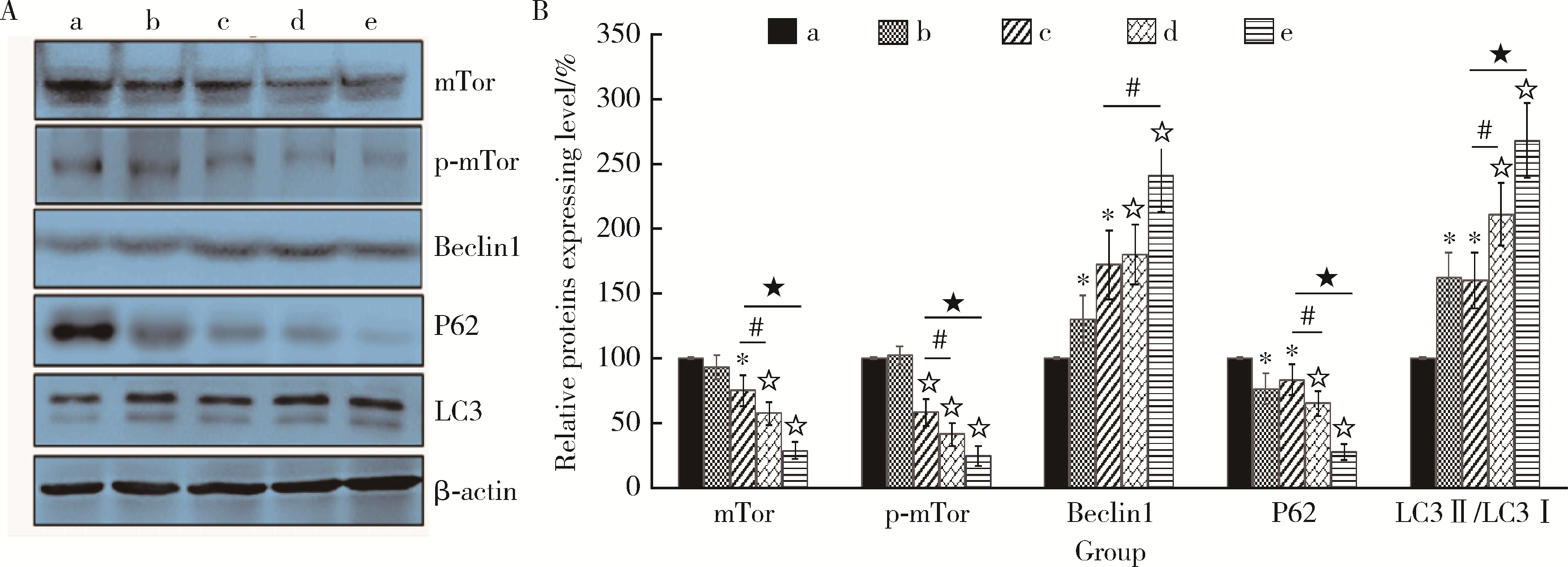
目的: 探讨姜黄素(curcumin,CUR)对锰中毒大鼠神经损伤的干预效应及其机制。方法: 成年雄性SD大鼠60只,随机分为5组,每组12只。(1)空白对照组: 通过腹腔注射(intraperitoneal injection,ip) 给予0.9%(质量分数)生理盐水,并通过灌胃(intragastric, ig)给予双蒸水(double distilled water,ddH2O);(2)锰中毒模型组: ip给予MnCl2 15 mg/kg(Mn2+ 6.48 mg/kg),ig给予ddH2O;(3)单独姜黄素组: ip给予0.9%生理盐水,ig给予CUR 300 mg/kg;(4)MnCl2+CUR1组:ip给予MnCl2 15 mg/kg,ig给予CUR 100 mg/kg;(5)MnCl2+CUR2组: ip给予MnCl215 mg/kg,ig给予CUR 300 mg/kg)。5组大鼠每周给药5 d, 连续给予4周。旷场实验、转棒实验检测动物的探索行为、焦虑抑郁状态、运动及平衡能力,Morris水迷宫(Morris water maze, MWM)实验检测动物的学习、记忆能力。每组6只大鼠进行脑纹状体HE染色和免疫组织化学(immunohistochemistry, IHC)检查,并且其中2只大鼠一侧纹状体进行透射电镜(transmission electron microscope,TEM)观察。每组另外6只大鼠的一侧纹状体利用电感耦合等离子体质谱(ICP-MS)测定锰含量,另一侧纹状体用免疫印迹(Western blotting, WB)检测α-突触核蛋白(α-Synuclein,α-Syn)、细胞质和细胞核转录因子EB(transcription factor EB,TFEB)、雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)、p-mTOR、Beclin1、P62和微管相关蛋白轻链蛋白3(microtubule-associated protein light chain-3,LC3)表达水平,脱氧核糖核苷酸末端转移酶介导的缺口末端标记法(terminal deoxynucleotidyl transterase-mediated dUTP nick end labeling; TUNEL)检测大鼠纹状体多巴胺能神经元凋亡。结果: 旷场和转棒实验中,锰中毒模型组水平运动距离、直立次数、中央区运动时间和运动距离、在棒时间、在棒圈数较空白对照组显著降低(P < 0.05),而MnCl2+CUR2组的上述指标均较锰中毒模型组显著增加(P < 0.05)。MWM定位航行实验第3、4天时,锰中毒模型组逃避潜伏期和游泳距离显著高于空白对照组,而MnCl2+CUR2组则显著高于锰中毒模型组(P < 0.05)。MWM探索实验中,锰中毒模型组大鼠穿越平台次数、目标象限游泳时间、目标象限游泳时间百分比、目标象限距离及目标象限距离百分比显著低于对照组(P < 0.05),而MnCl2+CUR2组大鼠这些指标均较锰中毒模型组显著增加(P < 0.05)。脑纹状体IHC和HE染色发现,锰中毒模型大鼠脑纹状体中酪氨酸羟化酶(tyrosine hydroxylase,TH)阳性多巴胺能神经细胞显著减少,嗜酸性细胞数量显著增加,MnCl2+CUR1和MnCl2+CUR2组TH阳性细胞显著增加,嗜酸性细胞减少,TEM观察发现,锰中毒模型组纹状体神经细胞出现染色质凝结,核固缩;线粒体水肿或空泡,可见溶酶体以及自噬泡。MnCl2+CUR2组染色质凝结,核固缩,线粒体水肿、空泡等均较锰中毒大鼠明显改善,并可见更多的溶酶体以及自噬泡。免疫印迹检测发现,锰中毒模型组α-Syn表达水平较空白对照组显著增加,而MnCl2+CUR2组聚集性α-Syn表达则较锰中毒模型组显著减少(P < 0.05)。TFEB及自噬相关蛋白免疫印迹检测发现,与空白对照组比较,单独CUR组、锰中毒模型组、MnCl2+CUR1和MnCl2+CUR2组纹状体胞核TFEB表达均显著增加(P < 0.05),与锰中毒模型组比较,MnCl2+CUR1和MnCl2+CUR2组其表达均显著增加(P < 0.05);与空白对照组比较,各组mTOR、p-mTOR、P62蛋白表达水平显著降低,Beclin1、LC3Ⅱ/LC3Ⅰ表达水平则显著增加(P < 0.05);与锰中毒模型组相比,MnCl2+CUR1和MnCl2+CUR2组大鼠脑纹状体中mTOR、p-mTOR及P62蛋白表达水平显著降低,Beclin1、LC3Ⅱ/LC3Ⅰ表达水平显著增加(P < 0.05)。纹状体TUNEL检测结果发现,与空白对照组比较,锰中毒模型组和MnCl2+CUR1组TUNEL阳性细胞显著增加(P < 0.05);而与锰中毒模型组相比,MnCl2+CUR2组TUNEL阳性细胞则显著降低(P < 0.05)。结论: 一定剂量的姜黄素干预可以减轻锰中毒大鼠纹状体多巴胺能神经元损伤,改善锰中毒大鼠的神经行为症状,减少α-Syn聚集,减少细胞凋亡,其可能的机制是通过促进TFEB核转位增强细胞自噬。
中图分类号:
- R135.1
| 1 | Tarale P, Chakrabarti T, Sivanesan S, et al. Potential role of epigenetic mechanism in manganese induced neurotoxicity[J/OL]. Biomed Res Int, 2016, 2016: 1-18(2016-05-26)[2022-02-01]. http://dx.doi.org/10.1155/2016/2548792. |
| 2 | Yu Q , Zhou YZ . High level of Mn in brain is a risk for Alzheimer disease[J]. Acta Physiologica Sinica, 2018, 70 (2): 193- 200. |
| 3 |
Michalke B , Fernsebner K . New insights into manganese toxicity and speciation[J]. J Trace Elem Med Biol, 2014, 28 (2): 106- 116.
doi: 10.1016/j.jtemb.2013.08.005 |
| 4 | 滕小华, 刘宇昊, 李克非, 等. 环境锰污染对生物健康的威胁[J]. 东北农业大学学报, 2021, 52 (1): 90- 96. |
| 5 |
Perl DP , Olanow CW . The neuropathology of manganese-induced Parkinsonism[J]. J Neuropathol Exp Neurol, 2007, 66 (8): 675- 682.
doi: 10.1097/nen.0b013e31812503cf |
| 6 |
窦长松, 智翠娜, 刘文丽, 等. 锰中毒和PD模型小鼠神经行为改变及损伤部位的比较研究[J]. 中华劳动卫生职业病杂志, 2018, 36 (2): 84- 90.
doi: 10.3760/cma.j.issn.1001-9391.2018.02.002 |
| 7 | 刘文丽, 窦长松, 王裕, 等. MnCl2和MPP+诱导SK-N-SH细胞氧化应激及自噬的比较[J]. 中华劳动卫生职业病杂志, 2017, 35 (2): 78- 82. |
| 8 |
Zhang HT , Mi L , Wang T , et al. PINK1/Parkin-mediated mitophagy play a protective role in manganese induced apoptosis in SH-SY5Y cells[J]. Toxicol In Vitro, 2016, 34, 212- 219.
doi: 10.1016/j.tiv.2016.04.006 |
| 9 |
Wang T , Li X , Yang D , et al. ER stress and ER stress-mediated apoptosis are involved in manganese-inducedneurotoxicity in the rat striatum in vivo[J]. Neurotoxicology, 2015, 48, 109- 119.
doi: 10.1016/j.neuro.2015.02.007 |
| 10 |
Settembre C , Zoncu R , Medina DL , et al. A lysosometo-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB[J]. EMBO J, 2012, 31 (5): 1095- 1108.
doi: 10.1038/emboj.2012.32 |
| 11 |
Sardiello M , Palmieri M , di Ronza A , et al. A gene network regulating lysosomal biogenesis and function[J]. Science, 2009, 325 (5939): 473- 477.
doi: 10.1126/science.1174447 |
| 12 |
Settembre C , Di Malta C , Polito VA , et al. TFEB links autophagy to lysosomal biogenesis[J]. Science, 2011, 332 (6036): 1429- 1433.
doi: 10.1126/science.1204592 |
| 13 |
Jaroonwitchawan T , Chaicharoenaudomrung N , Namkaew J , et al. Curcumin attenuates paraquat-induced cell death in human neuroblastoma cells through modulating oxidative stress and autophagy[J]. Neurosci Lett, 2017, 636, 40- 47.
doi: 10.1016/j.neulet.2016.10.050 |
| 14 |
Defazio R , Criado A , Zantedeschi V , et al. Neuroanatomy-based matrix-guided trimming protocol for the rat brain[J]. Toxicol Pathol, 2015, 43 (2): 249- 256.
doi: 10.1177/0192623314538345 |
| 15 |
Forouzanfar F , Read MI , Barreto GE , et al. Neuroprotective effects of curcumin through autophagy modulation[J]. IUBMB Life, 2020, 72 (4): 652- 664.
doi: 10.1002/iub.2209 |
| 16 |
Yi LT , Dong SQ , Wang SS , et al. Curcumin attenuates cognitive impairment by enhancing autophagy in chemotherapy[J]. Neurobiol Dis, 2020, 136, 104715.
doi: 10.1016/j.nbd.2019.104715 |
| 17 |
Ji HF , Shen L . The multiple pharmaceutical potential of curcumin in Parkinson's disease[J]. CNS Neurol Disord Drug Targets, 2014, 13 (2): 369- 373.
doi: 10.2174/18715273113129990077 |
| 18 |
Sharma N , Nehru B . Curcumin affords neuroprotection and inhibits α-Synuclein aggregation in lipopolysaccharide-induced Parkinson's disease model[J]. Inflammopharmacology, 2018, 26 (2): 349- 360.
doi: 10.1007/s10787-017-0402-8 |
| 19 |
Shen JD , Wei Y , Li YJ , et al. Curcumin reverses the depressive-like behavior and insulin resistance induced by chronic mild stress[J]. Metab Brain Dis, 2017, 32 (4): 1163- 1172.
doi: 10.1007/s11011-017-0017-1 |
| 20 | Liao D, Lv C, Cao L, et al. Curcumin attenuates chronic unpredictable mild stress-induced depressive-like behaviors via restoring changes in oxidative stress and the activation of Nrf2 signaling pathway in rats[J/OL]. Oxid Med Cell Longev, 2020, 2020: 1-11(2020-09-18)[2022-02-05]. https://doi.org/10.1155/2020/9268083. |
| 21 |
Mansouri Z , Sabetkasaei M , Moradi F , et al. Curcumin has neuroprotection effect on homocysteine rat model of Parkinson[J]. J Mol Neurosci, 2012, 47 (2): 234- 242.
doi: 10.1007/s12031-012-9727-3 |
| 22 | Maiti P , Dunbar GL . Use of curcumin, a natural polyphenol for targeting molecular pathways in treating age-related neurodegenerative diseases[J]. Int J Mol Sci, 2018, 19 (6): 16378. |
| 23 |
ELBini-Dhouib I , Doghri R , Ellefi A , et al. Curcumin attenuated neurotoxicity in sporadic animal model of Alzheimer's disease[J]. Molecules, 2021, 26 (10): 3011.
doi: 10.3390/molecules26103011 |
| 24 |
Verina T , Schneider JS , Guilarte TR . Manganese exposure induces α-synuclein aggregation in the frontal cortex of non-human primates[J]. Toxicol Lett, 2013, 217 (3): 177- 183.
doi: 10.1016/j.toxlet.2012.12.006 |
| 25 | 吴忧, 徐彬, 梁顺利, 等. 姜黄素对帕金森病小鼠运动障碍和多巴胺能神经元存活的影响及机制研究[J]. 中国中西医结合杂志, 2018, 38 (7): 838- 843. |
| 26 |
Singh PK , Kotia V , Ghosh D , et al. Curcumin modulates alpha-synuclein aggregation and toxicity[J]. ACS Chem Neurosci, 2013, 4 (3): 393- 407.
doi: 10.1021/cn3001203 |
| 27 |
Yu L , Chen Y , Tooze SA . Autophagy pathway: Cellular and molecular mechanisms[J]. Autophagy, 2018, 14 (2): 207- 215.
doi: 10.1080/15548627.2017.1378838 |
| 28 |
Satoo K , Noda NN , Kumeta H , et al. The structure of Atg4B-LC3 complex reveals the mechanism of LC3 processing and delipidation during autophagy[J]. EMBO J, 2009, 28 (9): 1341- 1350.
doi: 10.1038/emboj.2009.80 |
| 29 |
Zaffagnini G , Savova A , Danieli A , et al. Phasing out the bad-how SQSTM1/p62 sequesters ubiquitinated proteins for degradation by autophagy[J]. Autophagy, 2018, 14 (7): 1280- 1282.
doi: 10.1080/15548627.2018.1462079 |
| 30 |
Yoshii SR , Mizushima N . Monitoring and measuring autophagy[J]. Int J Mol Sci, 2017, 18 (9): 1865.
doi: 10.3390/ijms18091865 |
| 31 |
Zhang J , Cao R , Cai T , et al. The role of autophagy dysregulation in manganese-induced dopaminergic neurodegeneration[J]. Neurotox Res, 2013, 24 (4): 478- 490.
doi: 10.1007/s12640-013-9392-5 |
| 32 |
Limanaqi F , Biagioni F , Busceti CL , et al. Phytochemicals bridging autophagy induction and alpha-synuclein degradation in Parkinsonism[J]. Int J Mol Sci, 2019, 20 (13): 3274.
doi: 10.3390/ijms20133274 |
| [1] | 孔维恺忻,鄢尤奇,蔡文康,胡新. 十二烷基硫酸钠和吐温20复配体系对姜黄素的增溶和保护作用[J]. 北京大学学报(医学版), 2021, 53(1): 227-231. |
| [2] | 张帆,燕太强,郭卫. Rasfonin抑制骨肉瘤细胞143B的增殖和迁移[J]. 北京大学学报(医学版), 2019, 51(2): 234-238. |
| [3] | 孙静,宋卫东,闫思源,席志军. 氯喹抑制肾癌细胞活性促进舒尼替尼诱导的细胞凋亡[J]. 北京大学学报(医学版), 2018, 50(5): 778-784. |
| [4] | 曹珮,姜学军,席志军. 舒尼替尼通过抑制Akt/mTOR信号通路诱导肾癌细胞自噬[J]. 北京大学学报(医学版), 2016, 48(4): 584-589. |
| [5] | 杨轩, 袁栋栋, 姜学军, 席志军. 顺铂通过诱导膀胱癌细胞自噬促进细胞凋亡[J]. 北京大学学报(医学版), 2013, 45(2): 221-. |
|
||