北京大学学报(医学版) ›› 2022, Vol. 54 ›› Issue (5): 832-836. doi: 10.19723/j.issn.1671-167X.2022.05.009
中药抗病毒的物质基础:从五环三萜说开来
- 北京大学药学院,天然药物及仿生药物国家重点实验室,北京 100191
中图分类号:
- R932
| 1 |
Ruzicka L . The isoprene rule and the biogenesis of terpenic compounds[J]. Experientia, 1953, 9 (10): 357- 367.
doi: 10.1007/BF02167631 |
| 2 | Joshi RK . Role of natural products against microorganisms[J]. Am J Clin Microbiol Antimicrob, 2018, 1 (1): 1005. |
| 3 |
Papadopoulou K , Melton RE , Leggett M , et al. Compromised disease resistance in saponin-deficient plants[J]. Proc Natl Acad Sci USA, 1999, 96 (22): 12923- 12928.
doi: 10.1073/pnas.96.22.12923 |
| 4 |
Si L , Meng K , Tian Z , et al. Triterpenoids manipulate a broad range of virus-host fusion via wrapping the HR2 domain prevalent in viral envelopes[J]. Sci Adv, 2018, 4 (11): eaau8408.
doi: 10.1126/sciadv.aau8408 |
| 5 |
Yu M , Si L , Wang Y , et al. Discovery of pentacyclic triterpenoids as potential entry inhibitors of influenza viruses[J]. J Med Chem, 2014, 57 (23): 10058- 10071.
doi: 10.1021/jm5014067 |
| 6 |
Yu F , Wang Q , Zhang Z , et al. Development of oleanane-type triterpenes as a new class of HCV entry inhibitors[J]. J Med Chem, 2013, 56 (11): 4300- 4319.
doi: 10.1021/jm301910a |
| 7 |
Li H , Wang S , Ma W , et al. Discovery of pentacyclic triterpenoid PROTACs as a class of effective hemagglutinin protein degraders[J]. J Med Chem, 2022, 65 (10): 7154- 7169.
doi: 10.1021/acs.jmedchem.1c02013 |
| 8 |
DeJesus E , Harward S , Jewell RC , et al. A phase Ⅱ a study evaluating safety, pharmacokinetics, and antiviral activity of GSK2838232, a novel, second-generation maturation inhibitor, in participants with human immunodeficiency virus type 1 infection[J]. Clin Infect Dis, 2020, 71 (5): 1255- 1262.
doi: 10.1093/cid/ciz938 |
| 9 |
Margot NA , Gibbs CS , Miller MD . Phenotypic susceptibility to bevirimat in isolates from HIV-1-infected patients without prior exposure to bevirimat[J]. Antimicrob Agents Chemother, 2010, 54 (6): 2345- 2353.
doi: 10.1128/AAC.01784-09 |
| 10 |
Vigant F , Santos NC , Lee B . Broad-spectrum antivirals against viral fusion[J]. Nat Rev Microbiol, 2015, 13 (7): 426- 437.
doi: 10.1038/nrmicro3475 |
| 11 |
Harrison SC . Viral membrane fusion[J]. Nat Struct Mol Biol, 2008, 15 (7): 690- 698.
doi: 10.1038/nsmb.1456 |
| 12 |
Rennie ML , Chaugule VK , Walden H . Modes of allosteric regulation of the ubiquitination machinery[J]. Curr Opin Struct Biol, 2020, 62, 189- 196.
doi: 10.1016/j.sbi.2020.02.003 |
| 13 |
Weissenhorn W , Hinz A , Gaudin Y . Virus membrane fusion[J]. FEBS Lett, 2007, 581 (11): 2150- 2155.
doi: 10.1016/j.febslet.2007.01.093 |
| 14 |
Russell RJ , Gamblin SJ , Haire LF , et al. H1 and H7 influenza haemagglutinin structures extend a structural classification of haemagglutinin subtypes[J]. Virology, 2004, 325 (2): 287- 296.
doi: 10.1016/j.virol.2004.04.040 |
| 15 |
Bressanelli S , Stiasny K , Allison SL , et al. Structure of a flavivirus envelope glycoprotein in its low-pH-induced membrane fusion conformation[J]. Embo J, 2004, 23 (4): 728- 738.
doi: 10.1038/sj.emboj.7600064 |
| 16 |
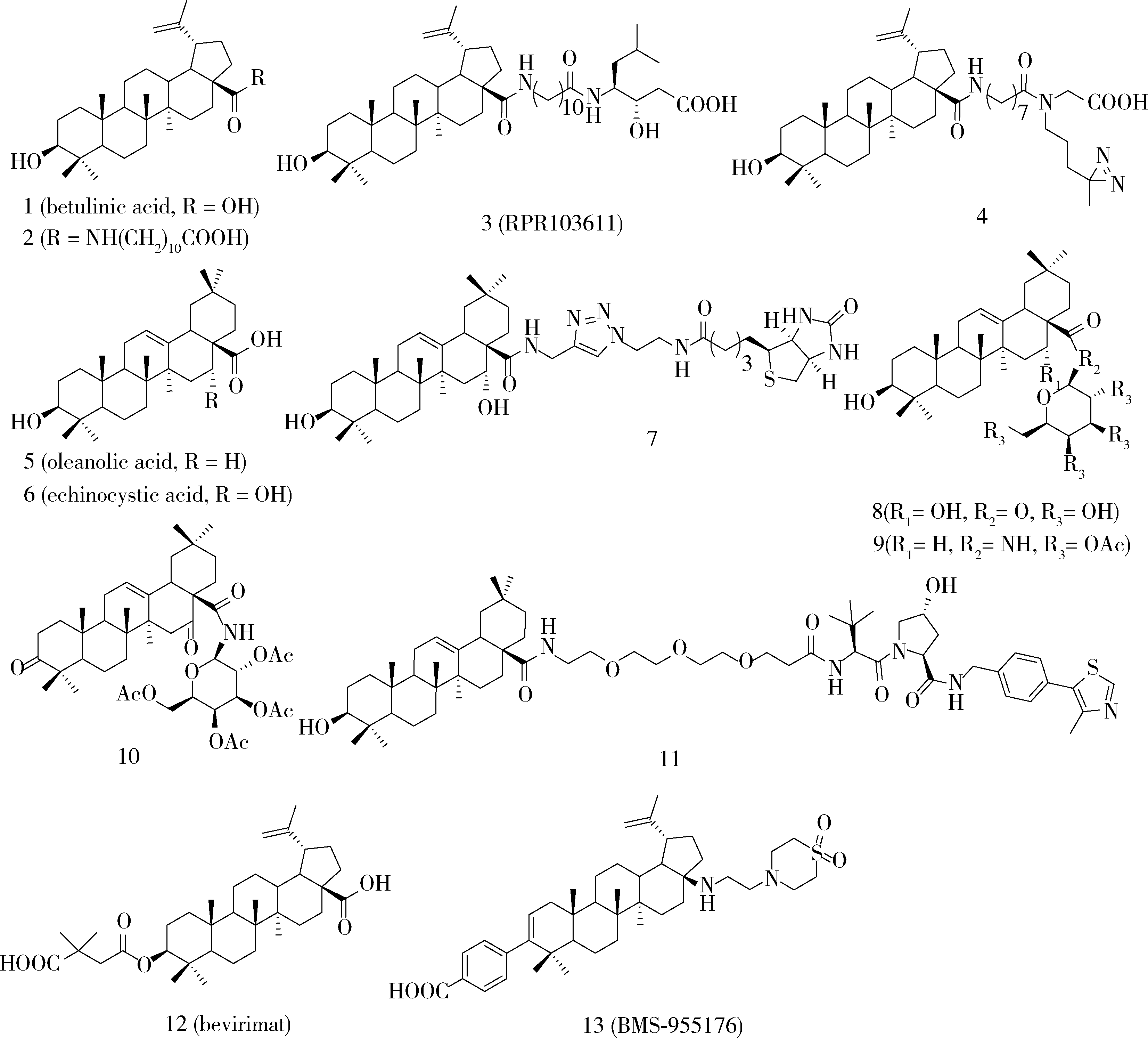
Fujioka T , Kashiwada Y , Kilkuskie RE , et al. Anti-AIDS agents, 11. betulinic acid and platanic acid as anti-HIV principles from Syzigium claviflorum, and the anti-HIV activity of structurally related triterpenoids[J]. J Nat Prod, 1994, 57 (2): 243- 247.
doi: 10.1021/np50104a008 |
| 17 |
Evers M , Poujade C , Soler F , et al. Betulinic acid derivatives: a new class of human immunodeficiency virus type 1 specific inhibitors with a new mode of action[J]. J Med Chem, 1996, 39 (5): 1056- 1068.
doi: 10.1021/jm950670t |
| 18 |
Kashiwada Y , Hashimoto F , Cosentino LM , et al. Betulinic acid and dihydrobetulinic acid derivatives as potent anti-HIV agents[J]. J Med Chem, 1996, 39 (5): 1016- 1017.
doi: 10.1021/jm950922q |
| 19 |
Mayaux JF , Bousseau A , Pauwels R , et al. Triterpene derivatives that block entry of human immunodeficiency virus type 1 into cells[J]. Proc Natl Acad Sci USA, 1994, 91 (9): 3564- 3568.
doi: 10.1073/pnas.91.9.3564 |
| 20 |
Dang Z , Lai W , Qian K , et al. Betulinic acid derivatives as human immunodeficiency virus type 2 (HIV-2) inhibitors[J]. J Med Chem, 2009, 52 (23): 7887- 7891.
doi: 10.1021/jm9004253 |
| 21 |
Soler F , Poujade C , Evers M , et al. Betulinic acid derivatives: a new class of specific inhibitors of human immunodeficiency virus type 1 entry[J]. J Med Chem, 1996, 39 (5): 1069- 1083.
doi: 10.1021/jm950669u |
| 22 | Yang JP , Zhou D , Wong-Staal F . Screening of small-molecule compounds as inhibitors of HCV entry[J]. Methods Mol Biol, 2009, 510, 295- 304. |
| 23 |
Côté M , Misasi J , Ren T , et al. Small molecule inhibitors reveal Niemann-Pick C1 is essential for Ebola virus infection[J]. Nature, 2011, 477 (7364): 344- 348.
doi: 10.1038/nature10380 |
| 24 |
Hu Y , Lu S , Song Z , et al. Association between adverse clinical outcome in human disease caused by novel influenza A H7N9 virus and sustained viral shedding and emergence of antiviral resistance[J]. Lancet, 2013, 381 (9885): 2273- 2279.
doi: 10.1016/S0140-6736(13)61125-3 |
| 25 |
Takashita E . Influenza polymerase inhibitors: mechanisms of action and resistance[J]. Cold Spring Harb Perspect Med, 2021, 11 (5): a038687.
doi: 10.1101/cshperspect.a038687 |
| 26 |
Burslem GM , Crews CM . Proteolysis-targeting chimeras as therapeutics and tools for biological discovery[J]. Cell, 2020, 181 (1): 102- 114.
doi: 10.1016/j.cell.2019.11.031 |
| 27 |
Chamberlain PP , Hamann LG . Development of targeted protein degradation therapeutics[J]. Nat Chem Biol, 2019, 15 (10): 937- 944.
doi: 10.1038/s41589-019-0362-y |
| 28 |
Bushweller JH . Targeting transcription factors in cancer: from undruggable to reality[J]. Nat Rev Cancer, 2019, 19 (11): 611- 624.
doi: 10.1038/s41568-019-0196-7 |
| 29 |
Li F , Goila-Gaur R , Salzwedel K , et al. PA-457: a potent HIV inhibitor that disrupts core condensation by targeting a late step in Gag processing[J]. Proc Natl Acad Sci USA, 2003, 100 (23): 13555- 13560.
doi: 10.1073/pnas.2234683100 |
| 30 |
Purdy MD , Shi D , Chrustowicz J , et al. MicroED structures of HIV-1 Gag CTD-SP1 reveal binding interactions with the maturation inhibitor bevi-rimat[J]. Proc Natl Acad Sci USA, 2018, 115 (52): 13258- 13263.
doi: 10.1073/pnas.1806806115 |
| 31 | Jacob J, Richards J, Augustine J, et al. Liquid bevirimat dosage forms for oral administration: WO2009042166A1[P/OL]. 2010-08-26[2022-08-20]. https://patents.google.com/patent/WO2009042166A1/en?oq=WO2009042166A1. |
| 32 |
Liu Z , Swidorski JJ , Nowicka-Sans B , et al. C-3 benzoic acid derivatives of C-3 deoxybetulinic acid and deoxybetulin as HIV-1 maturation inhibitors[J]. Bioorg Med Chem, 2016, 24 (8): 1757- 1770.
doi: 10.1016/j.bmc.2016.03.001 |
| 33 |
Swidorski JJ , Liu Z , Sit SY , et al. Inhibitors of HIV-1 maturation: development of structure-activity relationship for C-28 amides based on C-3 benzoic acid-modified triterpenoids[J]. Bioorg Med Chem Lett, 2016, 26 (8): 1925- 1930.
doi: 10.1016/j.bmcl.2016.03.019 |
| 34 |
Regueiro-Ren A , Dicker IB , Hanumegowda U , et al. Second generation inhibitors of HIV-1 maturation[J]. ACS Med Chem Lett, 2019, 10 (3): 287- 294.
doi: 10.1021/acsmedchemlett.8b00656 |
| 35 |
Regueiro-Ren A , Liu Z , Chen Y , et al. Discovery of BMS-955176, a second generation HIV-1 maturation inhibitor with broad spectrum antiviral activity[J]. ACS Med Chem Lett, 2016, 7 (6): 568- 572.
doi: 10.1021/acsmedchemlett.6b00010 |
| 36 |
Hwang C , Schürmann D , Sobotha C , et al. Antiviral activity, safety, and exposure-response relationships of GSK3532795, a second-generation human immunodeficiency virus type 1 maturation inhibitor, administered as monotherapy or in combination with atazanavir with or without ritonavir in a phase 2a randomized, dose-ranging, controlled trial (AI468002)[J]. Clin Infect Dis, 2017, 65 (3): 442- 452.
doi: 10.1093/cid/cix239 |
| [1] | 曹乐清,周婧睿,陈育红,陈欢,韩伟,陈瑶,张圆圆,闫晨华,程翼飞,莫晓冬,付海霞,韩婷婷,吕萌,孔军,孙于谦,王昱,许兰平,张晓辉,黄晓军. 异基因造血干细胞移植后晚发重症肺炎患者治疗与预后转归的关系[J]. 北京大学学报(医学版), 2022, 54(5): 1013-1020. |
| [2] | 刘文龙, 王路漫, 贺东奇, 张天蓝, 苟宝迪, 李庆. 寡糖分子结构及其分形[J]. 北京大学学报(医学版), 2014, 46(5): 739-743. |
| [3] | 杨秀伟, 杨晓达, 蒲小平, 钱忠明, 王夔. 创新药物研究中的吸收、分布、代谢、排泄/毒性(ADME/Tox.)平台建设[J]. 北京大学学报(医学版), 2004, 36(1): 5-8. |
| [4] | 杨秀伟, 张礼和. 基础和应用基础研究是创新药物研究与开发的源泉[J]. 北京大学学报(医学版), 2001, 33(3): 193-198. |
|
||