北京大学学报(医学版) ›› 2024, Vol. 56 ›› Issue (3): 519-525. doi: 10.19723/j.issn.1671-167X.2024.03.020
乳酸对类风湿关节炎患者外周血CD4+T细胞亚群的调控作用
黄会娜1,赵静2,赵祥格1,白自然1,李霞1,王冠1,*( )
)
- 1. 大连医科大学基础医学院免疫学教研室, 辽宁大连 116044
2. 北京大学人民医院风湿免疫科, 北京 100044
Regulatory effect of lactate on peripheral blood CD4+ T cell subsets in patients with rheumatoid arthritis
Huina HUANG1,Jing ZHAO2,Xiangge ZHAO1,Ziran BAI1,Xia LI1,Guan WANG1,*( )
)
- 1. Department of Immunology, College of Basic Medical Science, Dalian Medical University, Dalian 116044, Liaoning, China
2. Department of Rheumatology and Immunology, Peking University People's Hospital, Beijing 100044, China
摘要:
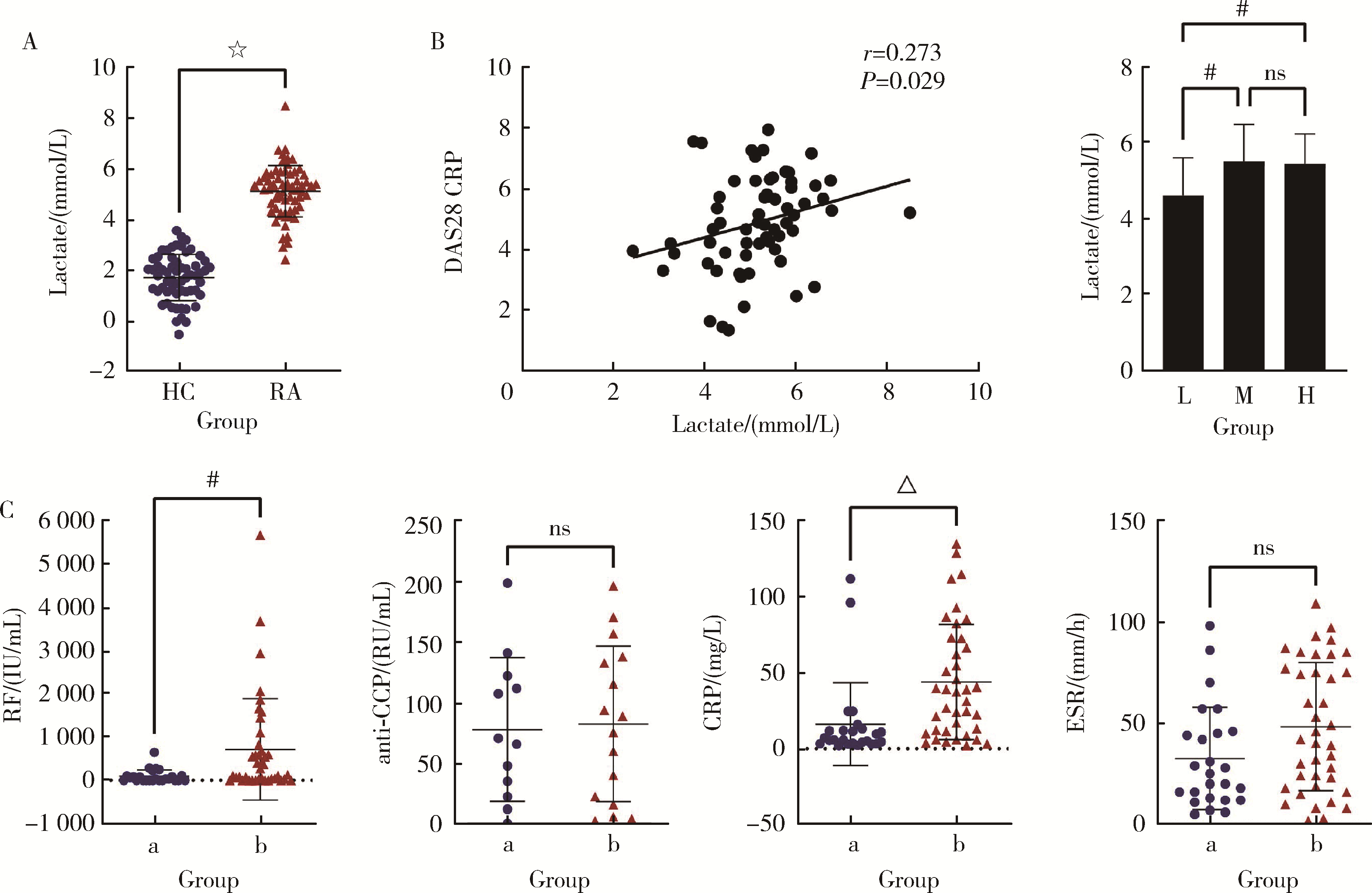
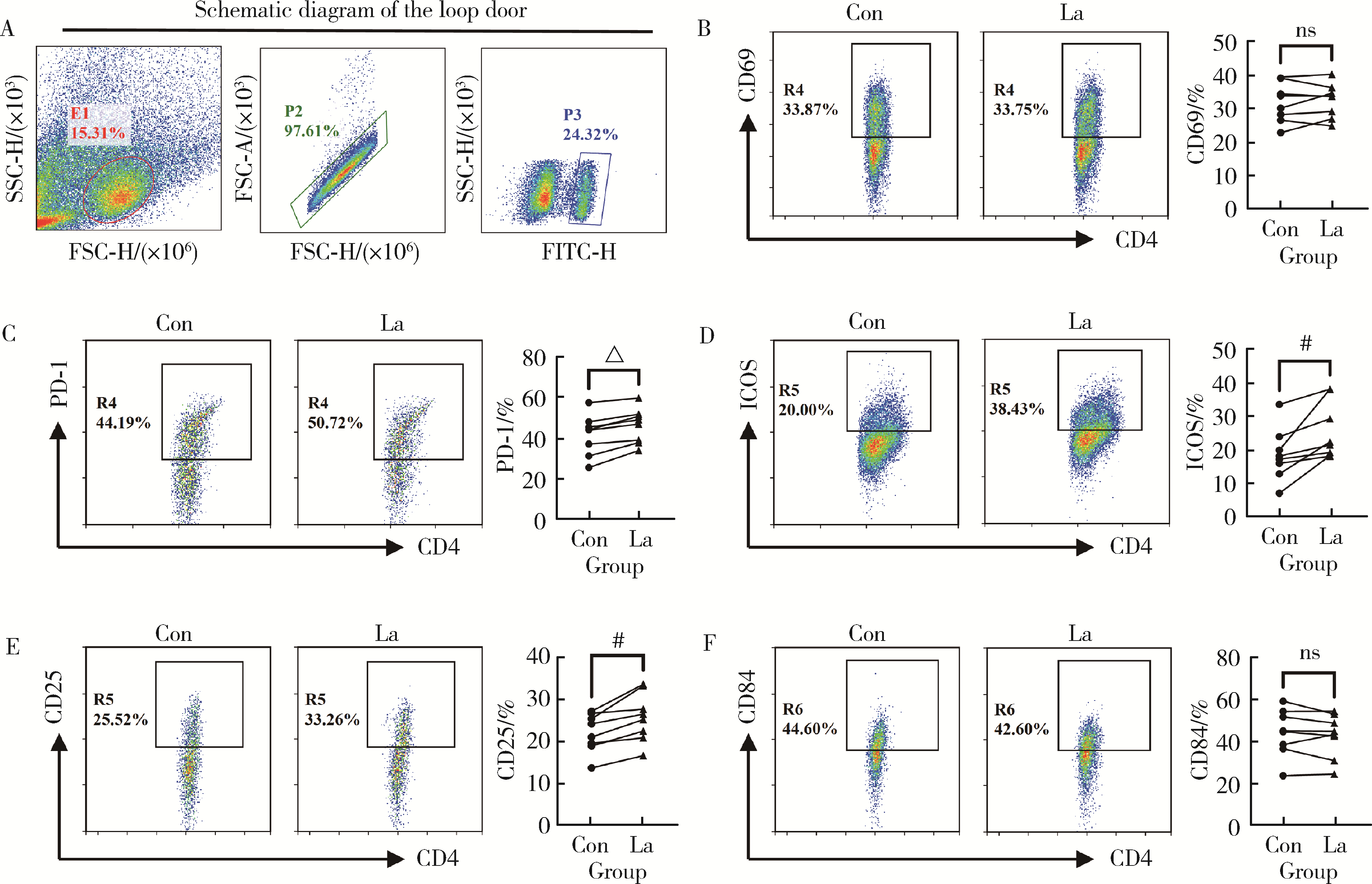
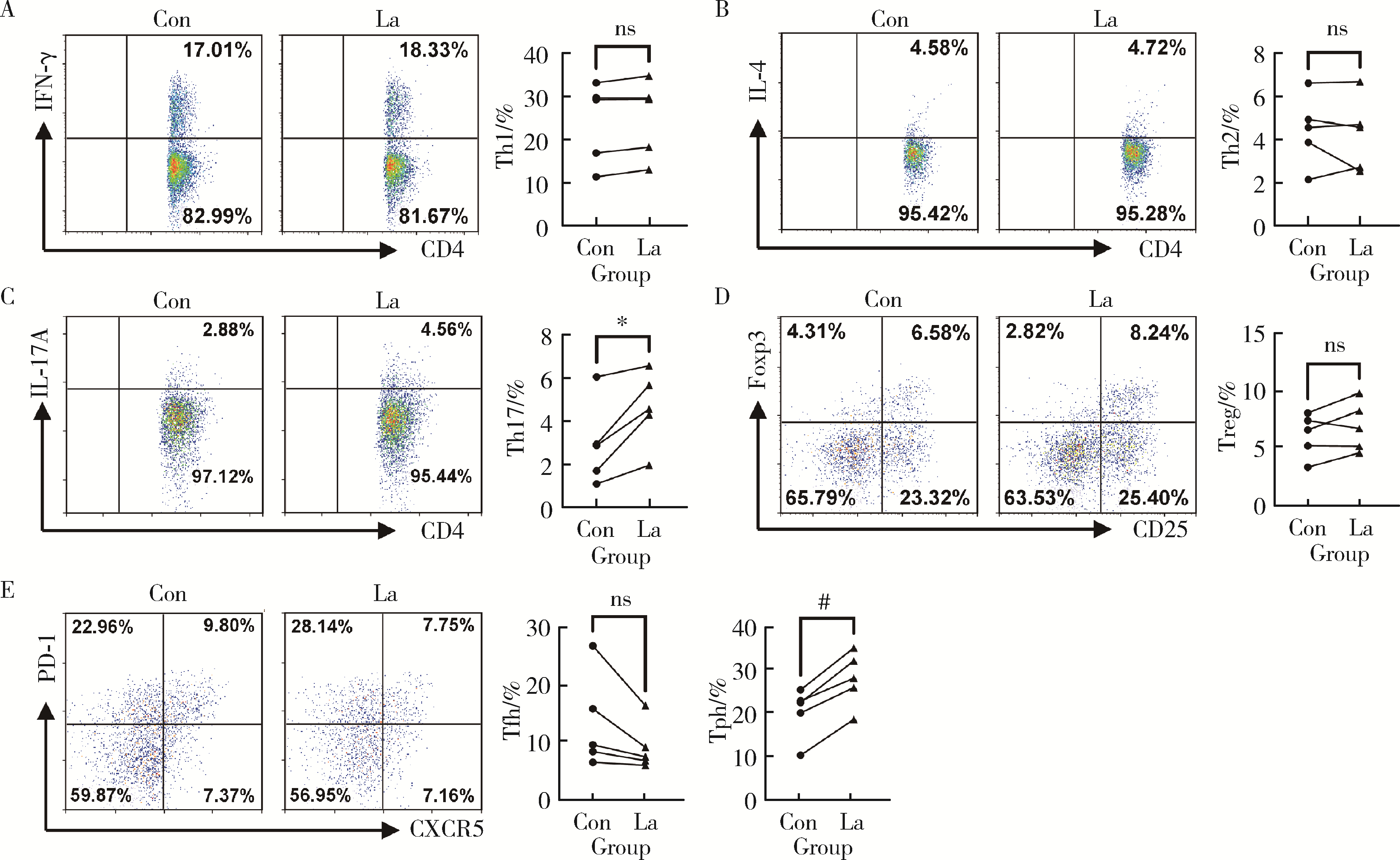
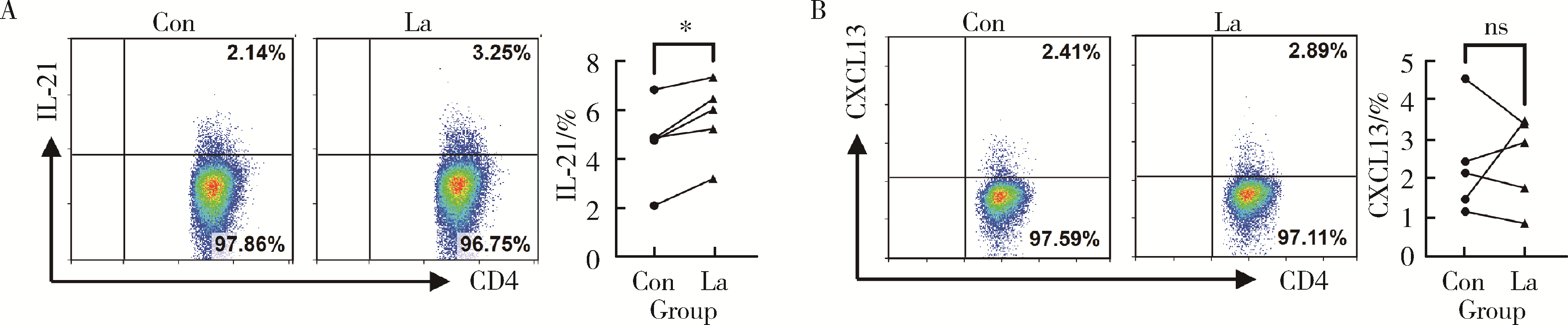
目的: 研究类风湿关节炎(rheumatoid arthritis, RA)患者血清乳酸水平及其与疾病活动度的关系,分析乳酸对RA患者外周血中CD4+T细胞的活化、分泌细胞因子能力以及对CD4+T细胞亚群的作用。方法: 选择大连医科大学附属第二医院风湿免疫科2019年10月至2021年9月连续收治的RA患者作为病例组,同期与RA患者性别年龄相匹配的大连医科大学附属第二医院体检中心健康人(healthy control, HC)作为对照组,收集两组外周血,乳酸检测试剂盒检测上清液中乳酸含量,并分析其与RA患者疾病评分的相关性。用乳酸刺激后,通过流式细胞术检测所有病例组RA患者外周血中CD4+T细胞的活化水平、CD4+T细胞亚群的比例以及CD4+T细胞分泌的细胞因子。结果: RA患者(n=66)的血清乳酸水平[(5.13±1.01) mmol/L vs. (1.72±0.91) mmol/L, P<0.001]明显高于HC(n=60),且与RA患者DAS28(disease activity score in 28 joints)-C反应蛋白(C-reactive protein,CRP)评分有相关性(r=0.273, P=0.029),乳酸浓度>5 mmol/L较浓度≤5 mmol/L的类风湿因子(rheumatoid factor, RF)[197.50 (26.03, 783.00) IU/mL vs. 29.30 (0.00, 102.60) IU/mL, P<0.01]和CRP [37.40 (11.30, 72.60) mg/L vs. 5.83 (2.36, 12.45) mg/L, P<0.001]的表达均升高,而红细胞沉降率(erythrocyte sedimentation rate, ESR)[42.00 (19.00, 77.00) mm/h vs. 25.00 (12.50, 45.50) mm/h, P>0.05]和抗环瓜氨酸多肽(cyclic citrullinated peptied, CCP)抗体[82.35 (17.70, 137.00) RU/mL vs. 68.60 (25.95, 119.70) RU/mL, P>0.05]的表达差异无统计学意义。与对照组HC相比,乳酸处理组的RA患者CD4+T细胞表面PD-1 (46.15%±8.54% vs. 41.67%±9.98%, P < 0.001)、诱导共刺激分子(inducible costimulatory molecule, ICOS)(25.77%±8.60% vs. 18.65%±7.94%, P < 0.01)和CD25 (25.89%±5.80% vs. 22.25%±4.59%, P < 0.01)的表达明显升高;乳酸处理组的RA患者CD4+T细胞中Th17 (4.62%±1.74% vs. 2.93%±1.92%, P < 0.05)、外周辅助性T (peripheral helper T, Tph)细胞(28.02%±6.28% vs. 20.32%±5.82%, P < 0.01)的比例升高;乳酸处理组的RA患者CD4+T细胞中IL-21(5.73%±1.59% vs. 4.75%±1.71%, P < 0.05)的表达上调。结论: RA患者血清乳酸水平升高,促进了RA患者CD4+T细胞活化和分泌IL-21,以及上调了RA患者Th17、Tph细胞的比例。
中图分类号:
- R593.22
| 1 |
Ding Q , Hu W , Wang R , et al. Signaling pathways in rheumatoid arthritis: Implications for targeted therapy[J]. Signal Transduct Target Ther, 2023, 8 (1): 68.
doi: 10.1038/s41392-023-01331-9 |
| 2 |
McInnes IB , Schett G . The pathogenesis of rheumatoid arthritis[J]. N Engl J Med, 2011, 365 (23): 2205- 2219.
doi: 10.1056/NEJMra1004965 |
| 3 |
Chen W , Yu Y , Zheng SG , et al. Human gingival tissue-derived mesenchymal stem cells inhibit proliferation and invasion of rheumatoid fibroblast-like synoviocytes via the CD39/CD73 signaling pathway[J]. Rheumatol Autoimmun, 2023, 3, 90- 99.
doi: 10.1002/rai2.12075 |
| 4 | Wang D , Li Y , Liu Y , Shi G . The role of autoreactive T cell in the pathogenesis of rheumatoid arthritis and implications for T cell targeted vaccine therapy[J]. Minerva Med, 2015, 106 (3): 157- 167. |
| 5 |
Yamada H . The search for the pathogenic T cells in the joint of rheumatoid arthritis: Which T-cell subset drives autoimmune inflammation[J]. Int J Mol Sci, 2023, 24 (8): 6930.
doi: 10.3390/ijms24086930 |
| 6 |
Chemin K , Gerstner C , Malmström V . Effector functions of CD4+ T cells at the site of local autoimmune inflammation: Lessons from rheumatoid arthritis[J]. Front Immunol, 2019, 10, 353.
doi: 10.3389/fimmu.2019.00353 |
| 7 |
Yoshitomi H . Peripheral helper T cell responses in human diseases[J]. Front Immunol, 2022, 13, 946786.
doi: 10.3389/fimmu.2022.946786 |
| 8 |
Lucas C , Perdriger A , Amé P . Definition of B cell helper T cells in rheumatoid arthritis and their behavior during treatment[J]. Semin Arthritis Rheum, 2020, 50 (5): 867- 872.
doi: 10.1016/j.semarthrit.2020.06.021 |
| 9 |
Huang Y , Ba X , Han L , et al. T peripheral helper cells in autoimmune diseases: What do we know[J]. Front Immunol, 2023, 14, 1145573.
doi: 10.3389/fimmu.2023.1145573 |
| 10 | Sun S , Li H , Chen J , et al. Lactic acid: No longer an inert and end-product of glycolysis[J]. Physiology (Bethesda), 2017, 32 (6): 453- 463. |
| 11 |
Ye L , Jiang Y , Zhang M . Crosstalk between glucose metabolism, lactate production and immune response modulation[J]. Cytokine Growth Factor Rev, 2022, 68, 81- 92.
doi: 10.1016/j.cytogfr.2022.11.001 |
| 12 |
段姣妞, 张改连. 乳酸在类风湿关节炎发病机制及治疗中的研究进展[J]. 中华风湿病学杂志, 2022, 26 (12): 842- 845.
doi: 10.3760/cma.j.cn141217-20220323-00104 |
| 13 |
Haas R , Smith J , Rocher-Ros V , et al. Lactate regulates metabolic and pro-inflammatory circuits in control of T cell migration and effector functions[J]. PLoS Biol, 2015, 13 (7): e1002202.
doi: 10.1371/journal.pbio.1002202 |
| 14 |
Pucino V , Certo M , Bulusu V , et al. Lactate buildup at the site of chronic inflammation promotes disease by inducing CD4(+) T cell metabolic rewiring[J]. Cell Metab, 2019, 30 (6): 1055- 1074. e8.
doi: 10.1016/j.cmet.2019.10.004 |
| 15 |
Pucino V , Bombardieri M , Pitzalis C , et al. Lactate at the crossroads of metabolism, inflammation, and autoimmunity[J]. Eur J Immunol, 2017, 47 (1): 14- 21.
doi: 10.1002/eji.201646477 |
| 16 |
Chang X , Wei C . Glycolysis and rheumatoid arthritis[J]. Int J Rheum Dis, 2011, 14 (3): 217- 222.
doi: 10.1111/j.1756-185X.2011.01598.x |
| 17 | Wang Q , Asenso J , Xiao N , et al. Lactic acid regulation: A potential therapeutic option in rheumatoid arthritis[J]. J Immunol Res, 2022, 2022, 2280973. |
| 18 |
Rao DA , Gurish MF , Marshall JL , et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis[J]. Nature, 2017, 542 (7639): 110- 114.
doi: 10.1038/nature20810 |
| 19 |
Marks KE , Rao DA . T peripheral helper cells in autoimmune diseases[J]. Immunol Rev, 2022, 307 (1): 191- 202.
doi: 10.1111/imr.13069 |
| [1] | 刘东武, 陈杰, 高明利, 于静. 类风湿关节炎伴发淋巴结Castleman样病理改变1例[J]. 北京大学学报(医学版), 2024, 56(5): 928-931. |
| [2] | 汤晓菲,李永红,丁秋玲,孙卓,张阳,王育梅,田美伊,刘坚. 类风湿关节炎患者下肢深静脉血栓发病率及危险因素[J]. 北京大学学报(医学版), 2024, 56(2): 279-283. |
| [3] | 邹雪,白小娟,张丽卿. 艾拉莫德联合托法替布治疗难治性中重度类风湿关节炎的疗效[J]. 北京大学学报(医学版), 2023, 55(6): 1013-1021. |
| [4] | 吴琦,蔡月明,何娟,黄文蒂,王庆文. 血脂异常与类风湿关节炎肺间质病变的相关性分析[J]. 北京大学学报(医学版), 2023, 55(6): 982-992. |
| [5] | 张警丰,金银姬,魏慧,姚中强,赵金霞. 体重指数与类风湿关节炎临床特征的相关性分析[J]. 北京大学学报(医学版), 2023, 55(6): 993-999. |
| [6] | 金银姬,孙琳,赵金霞,刘湘源. 血清IgA型抗鼠科肉瘤病毒癌基因同源物B1抗体在类风湿关节炎中的意义[J]. 北京大学学报(医学版), 2023, 55(4): 631-635. |
| [7] | 蔡文心,李仕成,刘一鸣,梁如玉,李静,郭建萍,胡凡磊,孙晓麟,李春,刘栩,叶华,邓立宗,李茹,栗占国. 类风湿关节炎临床分层及其特征的横断面研究[J]. 北京大学学报(医学版), 2022, 54(6): 1068-1073. |
| [8] | 程昉,杨邵英,房星星,王璇,赵福涛. CCL28-CCR10通路在类风湿关节炎单核细胞迁移中的作用[J]. 北京大学学报(医学版), 2022, 54(6): 1074-1078. |
| [9] | 刘蕊,赵金霞,闫良. 类风湿关节炎合并下肢静脉血栓患者的临床特点[J]. 北京大学学报(医学版), 2022, 54(6): 1079-1085. |
| [10] | 张警丰,金银姬,魏慧,姚中强,赵金霞. 类风湿关节炎患者生活质量与疾病活动度的横断面研究[J]. 北京大学学报(医学版), 2022, 54(6): 1086-1093. |
| [11] | 高超,陈立红,王莉,姚鸿,黄晓玮,贾语博,刘田. 类风湿关节炎合并纤维肌痛简易分类标准的临床验证[J]. 北京大学学报(医学版), 2022, 54(2): 278-282. |
| [12] | 娄雪,廖莉,李兴珺,王楠,刘爽,崔若玫,徐健. 类风湿关节炎患者外周血TWEAK基因启动子区甲基化状态及其表达[J]. 北京大学学报(医学版), 2021, 53(6): 1020-1025. |
| [13] | 钟华,徐丽玲,白明欣,苏茵. 类风湿关节炎患者趋化因子CXCL9和CXCL10在骨侵蚀中的作用[J]. 北京大学学报(医学版), 2021, 53(6): 1026-1031. |
| [14] | 罗靓,霍文岗,张钦,李春. 类风湿关节炎合并角膜溃疡的临床特点和相关因素分析[J]. 北京大学学报(医学版), 2021, 53(6): 1032-1036. |
| [15] | 张璐,胡小红,陈澄,蔡月明,王庆文,赵金霞. 类风湿关节炎初治患者颈椎失稳情况及临床特征[J]. 北京大学学报(医学版), 2021, 53(6): 1049-1054. |
|
||