北京大学学报(医学版) ›› 2024, Vol. 56 ›› Issue (5): 756-762. doi: 10.19723/j.issn.1671-167X.2024.05.002
卵清蛋白诱导的特应性皮炎小鼠模型中白细胞介素-25的作用及其调控意义
- 北京大学人民医院皮肤科,北京 100044
The role and its regulatory significance of interleukin-25 in ovalbumin induced atopic dermatitis of mice
Jiang JIN*( ), Xue CHEN, Yan ZHAO, Jun JIA, Jianzhong ZHANG
), Xue CHEN, Yan ZHAO, Jun JIA, Jianzhong ZHANG
- Department of Dermatology, Peking University People's Hospital, Beijing 100044, China
摘要:
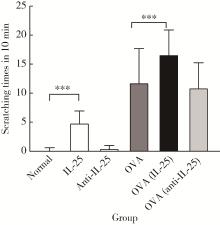
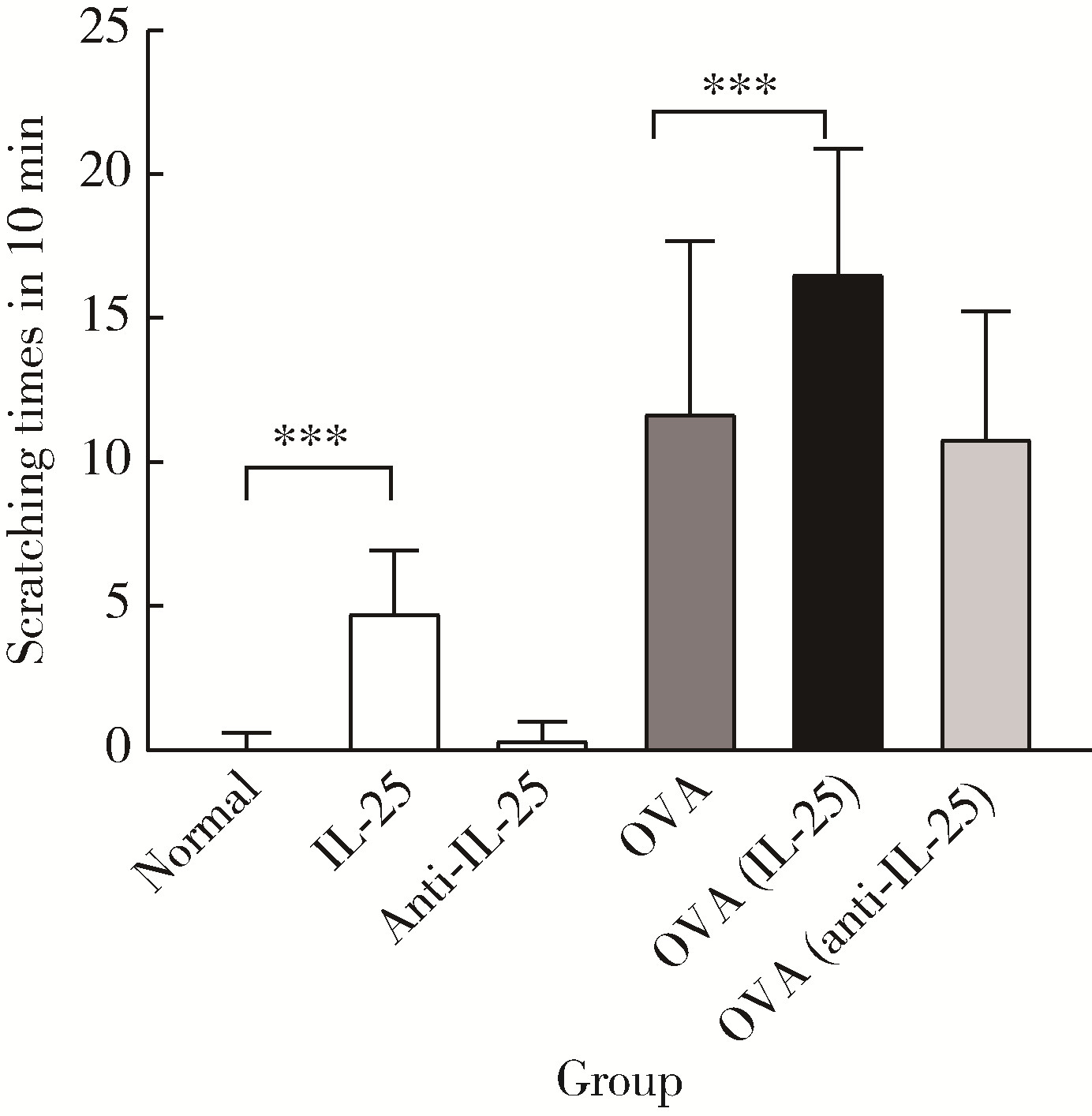
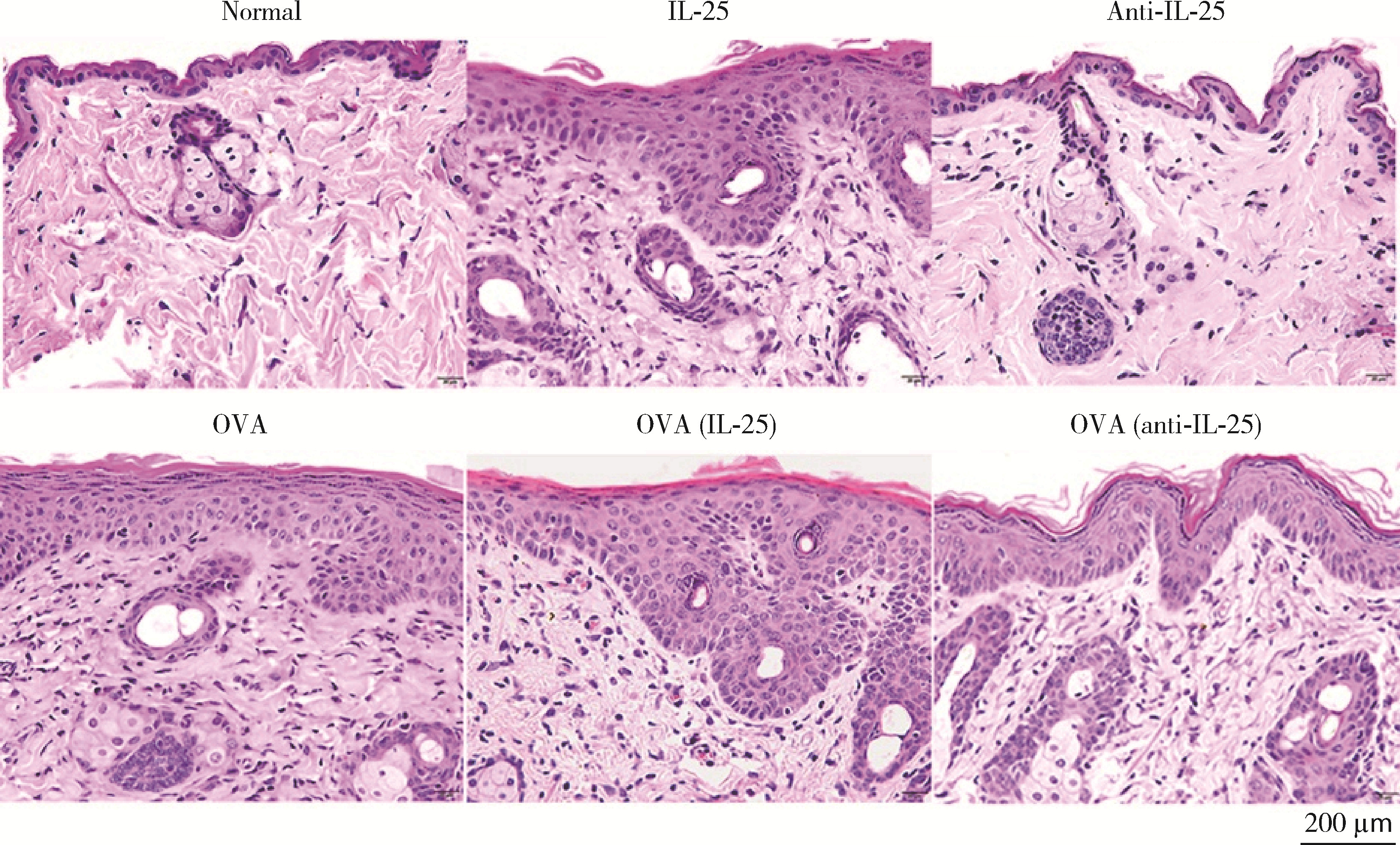
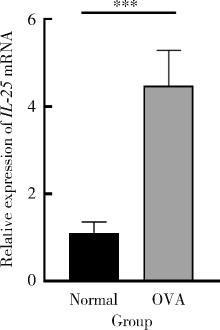
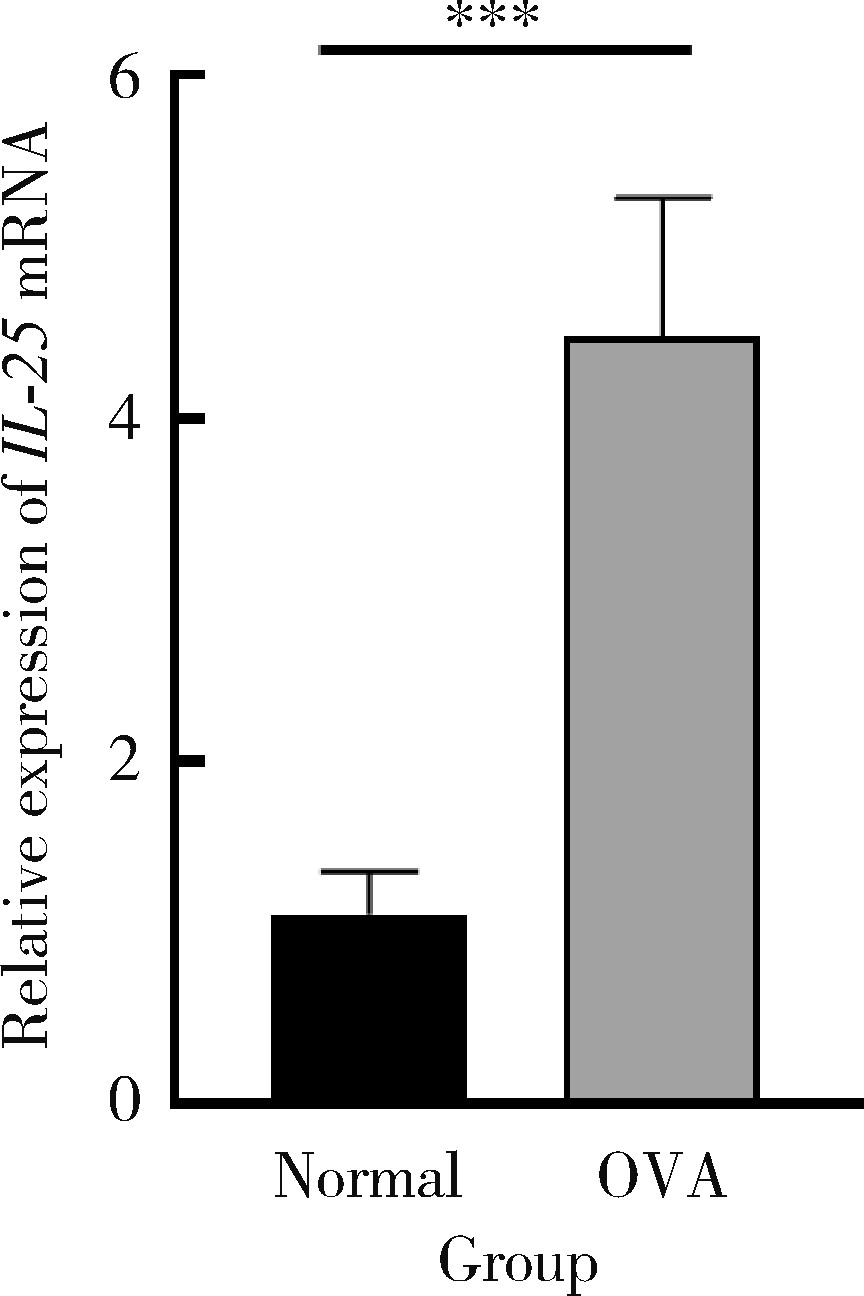
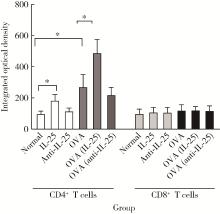
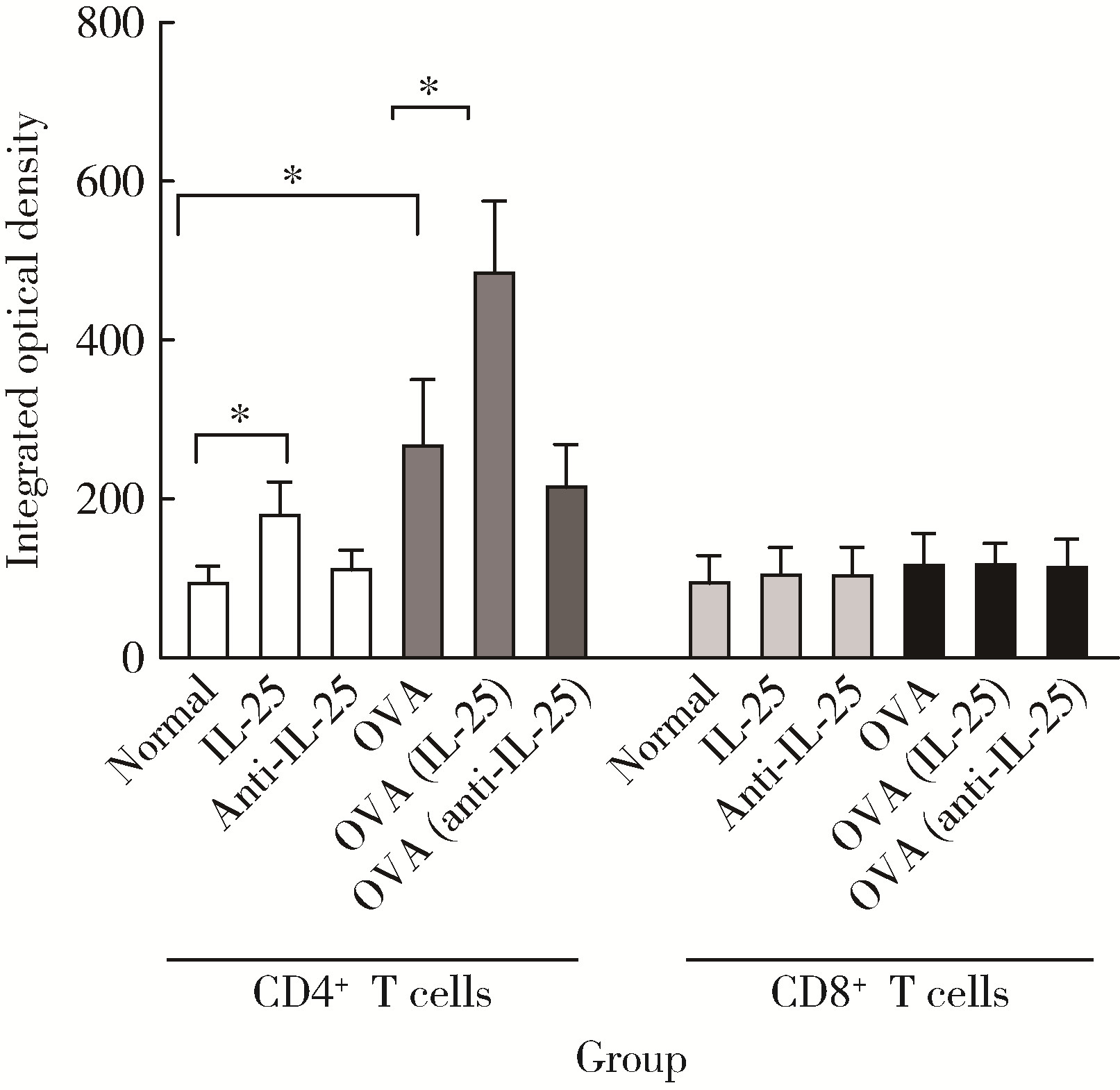
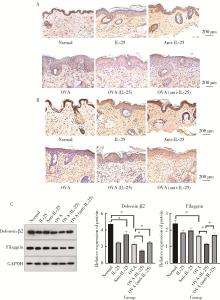
目的: 探讨白细胞介素-25(interleukin-25,IL-25)对卵清蛋白(ovalbumin,OVA)诱导的特应性皮炎小鼠模型的影响,以及调控IL-25的意义。方法: 将90只健康雄性6周龄无特定病原体(specific pathogen free, SPF)级BALB/c小鼠分为6组(每组15只),分别为: ①皮下注射磷酸盐缓冲液(phosphate buffered saline, PBS)组(正常对照组); ②皮下注射小鼠IL-25组(IL-25组); ③皮下注射抗小鼠IL-25单克隆抗体组(anti-IL-25组),每日皮下注射1次×1周,间隔2周,重复每日皮下注射1次×1周,间隔2周,再重复每日皮下注射1次×1周,总共7周; ④ OVA致敏组(模型组); ⑤ OVA致敏及IL-25皮下注射组(IL-25干预致敏组); ⑥ OVA致敏及anti-IL-25注射组(anti-IL-25干预致敏组)。⑤⑥组在致敏过程中给予IL-25或anti-IL-25的方式同②③组。致敏期间观察比较小鼠的搔抓行为和皮肤表现,致敏结束24 h后由小鼠心脏取血,分离血清,采用酶联免疫吸附试验(enzyme linked immunosorbent assay, ELISA)检测总IgE、IL-4、IL-5、IL-13等。取致敏部位的皮肤进行苏木精-伊红(hematoxylin-eosin,HE)染色、免疫组织化学、实时PCR(real-time PCR)及Western blot检测。采用单因素(ANOVA)方差分析比较各组间各项指标的差异。结果: 实验小鼠末次处理24 h后,IL-25干预致敏组的搔抓次数高于模型组,anti-IL-25干预致敏组的搔抓行为显著低于模型组; IL-25干预致敏组特应性皮炎表现、表皮增厚及真皮炎细胞浸润程度均明显重于模型组及anti-IL-25干预致敏组; IL-25干预致敏组血清IgE、IL-4、IL-5、IL-13水平显著高于模型组及anti-IL-25干预致敏组; IL-25干预致敏组CD4+ T细胞在真皮层较anti-IL-25干预致敏组显著增多; IL-25干预致敏组的丝聚蛋白(filaggrin)及防御素β2(defensin β2)蛋白水平明显低于模型组或anti-IL-25干预致敏组。结论: 在OVA诱导的皮炎模型中,IL-25能够明显促进小鼠表皮屏障功能损害,加重OVA诱导的皮炎损害,拮抗IL-25可一定程度上缓解OVA诱导的皮炎损害。
中图分类号:
- R758.2
| 1 |
Barbarot S , Auziere S , Gadkari A , et al. Epidemiology of atopic dermatitis in adults: Results from an international survey[J]. Allergy, 2018, 73 (6): 1284- 1293.
doi: 10.1111/all.13401 |
| 2 |
Aktar MK , Kido-Nakahara M , Furue M , et al. Mutual upregulation of endothelin-1 and IL-25 in atopic dermatitis[J]. Allergy, 2015, 70 (7): 846- 854.
doi: 10.1111/all.12633 |
| 3 |
Fort MM , Cheung J , Yen D , et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo[J]. Immunity, 2001, 15 (6): 985- 995.
doi: 10.1016/S1074-7613(01)00243-6 |
| 4 |
Suto H , Nambu A , Morita H , et al. IL-25 enhances TH17 cell-mediated contact dermatitis by promoting IL-1β production by dermal dendritic cells[J]. J Allergy Clin Immunol, 2018, 142 (5): 1500- 1509.e10.
doi: 10.1016/j.jaci.2017.12.1007 |
| 5 |
Senra L , Mylonas A , Kavanagh RD , et al. IL-17E (IL-25) enhances innate immune responses during skin inflammation[J]. J Invest Dermatol, 2019, 139 (8): 1732- 1742.e17.
doi: 10.1016/j.jid.2019.01.021 |
| 6 |
Tong X , Li B . A role of IL-25, a sibling of IL-17, in triggering psoriatic skin inflammation[J]. Sci China Life Sci, 2018, 61 (11): 1437- 1438.
doi: 10.1007/s11427-018-9330-x |
| 7 |
Hagner SL , Harb H , Zhao M , et al. Farm-derived Gram-positive bacterium Staphylococcus sciuri W620 prevents asthma phenotype in HDM- and OVA-exposed mice[J]. Allergy, 2013, 68 (3): 322- 329.
doi: 10.1111/all.12094 |
| 8 |
Orciani M , Campanati A , Caffarini M , et al. T helper (Th)1, Th17 and Th2 imbalance in mesenchymal stem cells of adult patients with atopic dermatitis: At the origin of the problem[J]. Br J Dermatol, 2017, 176 (6): 1569- 1576.
doi: 10.1111/bjd.15078 |
| 9 |
Mashiko S , Mehta H , Bissonnette R , et al. Increased frequencies of basophils, type 2 innate lymphoid cells and Th2 cells in skin of patients with atopic dermatitis but not psoriasis[J]. J Dermatol Sci, 2017, 88 (2): 167- 174.
doi: 10.1016/j.jdermsci.2017.07.003 |
| 10 |
Furue M , Chiba T , Tsuji G , et al. Atopic dermatitis: Immune deviation, barrier dysfunction, IgE autoreactivity and new therapies[J]. Allergol Int, 2017, 66 (3): 398- 403.
doi: 10.1016/j.alit.2016.12.002 |
| 11 |
Kumar A , Rani L , Mhaske ST , et al. IL-3 receptor expression on activated human Th cells is regulated by IL-4, and IL-3 synergizes with IL-4 to enhance Th2 cell differentiation[J]. J Immunol, 2020, 204 (4): 819- 831.
doi: 10.4049/jimmunol.1801629 |
| 12 |
Ochiai S , Jagot F , Kyle RL , et al. Thymic stromal lymphopoietin drives the development of IL-13+ Th2 cells[J]. Proc Natl Acad Sci USA, 2018, 115 (5): 1033- 1038.
doi: 10.1073/pnas.1714348115 |
| 13 |
Coomes SM , Kannan Y , Pelly VS , et al. CD4+ Th2 cells are directly regulated by IL-10 during allergic airway inflammation[J]. Mucosal Immunol, 2017, 10 (1): 150- 161.
doi: 10.1038/mi.2016.47 |
| 14 |
Prout MS , Kyle RL , Ronchese F , et al. IL-4 is a key requirement for IL-4- and IL-4/IL-13-expressing CD4 Th2 subsets in lung and skin[J]. Front Immunol, 2018, 9, 1211.
doi: 10.3389/fimmu.2018.01211 |
| 15 |
Hönzke S , Wallmeyer L , Ostrowski A , et al. Influence of Th2 cytokines on the cornified envelope, tight junction proteins, and β-defensins in filaggrin-deficient skin equivalents[J]. J Invest Dermatol, 2016, 136 (3): 631- 639.
doi: 10.1016/j.jid.2015.11.007 |
| 16 |
Drislane C , Irvine AD . The role of filaggrin in atopic dermatitis and allergic disease[J]. Ann Allergy Asthma Immunol, 2020, 124 (1): 36- 43.
doi: 10.1016/j.anai.2019.10.008 |
| 17 |
Wallmeyer L , Dietert K , Sochorová M , et al. TSLP is a direct trigger for T cell migration in filaggrin-deficient skin equivalents[J]. Sci Rep, 2017, 7 (1): 774.
doi: 10.1038/s41598-017-00670-2 |
| 18 | Christian L , Gerardus B , Marie-Anne V , et al. Novel microperfusion method confirms higher IL-17A and β-defensin-2 levels in psoriasis lesional skin compared to non-lesional skin[J]. J Dermatol Sci, 2016, 84 (1): 32- 33. |
| [1] | 胡宇晴,张建中. 156例特应性皮炎患者血清吸入和食物过敏原特异性免疫球蛋白E及患者自觉过敏情况[J]. 北京大学学报(医学版), 2020, 52(5): 980-984. |
| [2] | 白珊珊,莫思怡,徐啸翔,刘云,谢秋菲,曹烨. 大鼠咬合干扰致口颌面痛敏的自我赏罚实验行为学特点[J]. 北京大学学报(医学版), 2020, 52(1): 51-57. |
|
||