北京大学学报(医学版) ›› 2024, Vol. 56 ›› Issue (5): 839-844. doi: 10.19723/j.issn.1671-167X.2024.05.014
中国健康成人外周血自然杀伤细胞及其亚群的正常值范围流式细胞学分析
- 北京大学人民医院风湿免疫科,北京 100040
Flow cytometry analysis of normal range of natural killer cells and their subsets in peripheral blood of healthy Chinese adults
Jiayi TIAN, Yixue GUO, Xia ZHANG, Xiaolin SUN, Jing HE*( )
)
- Department of Rheumatology and Immunology, Peking University People's Hospital, Beijing 100044, China
摘要:
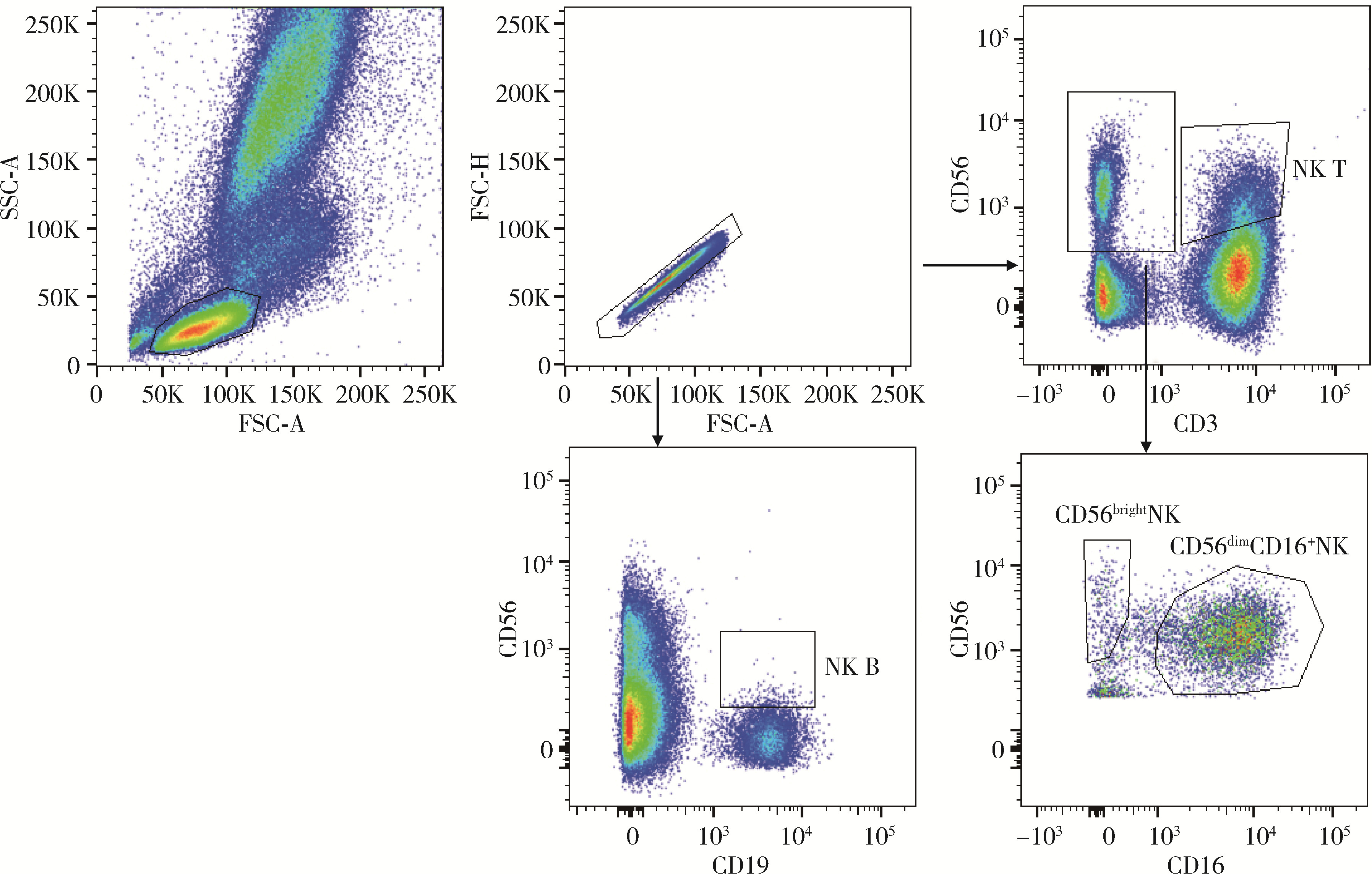
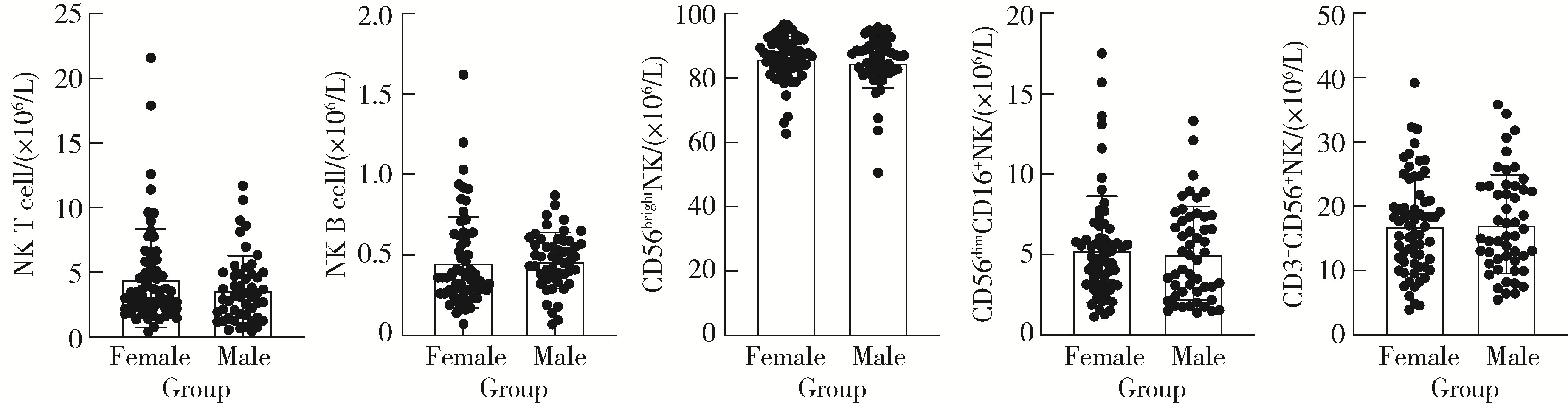
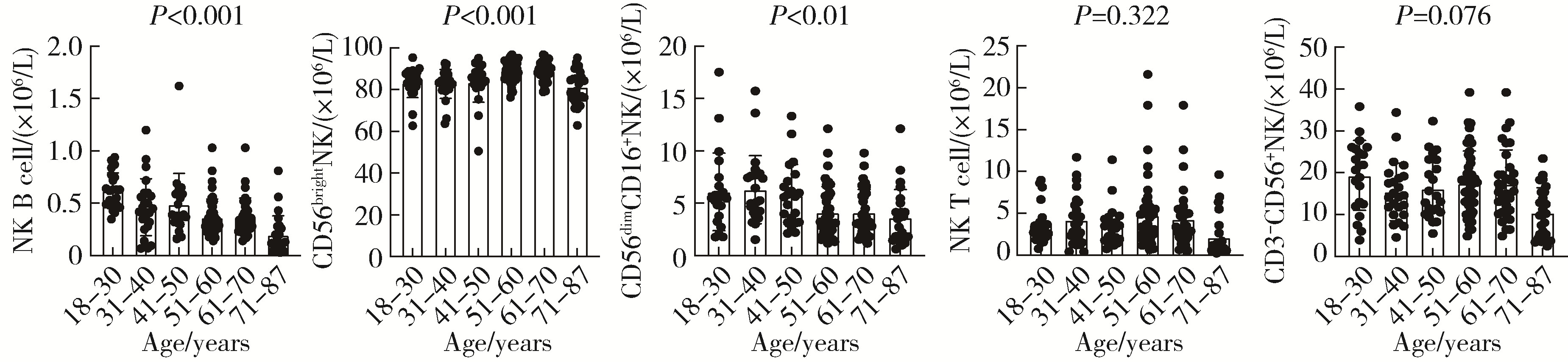
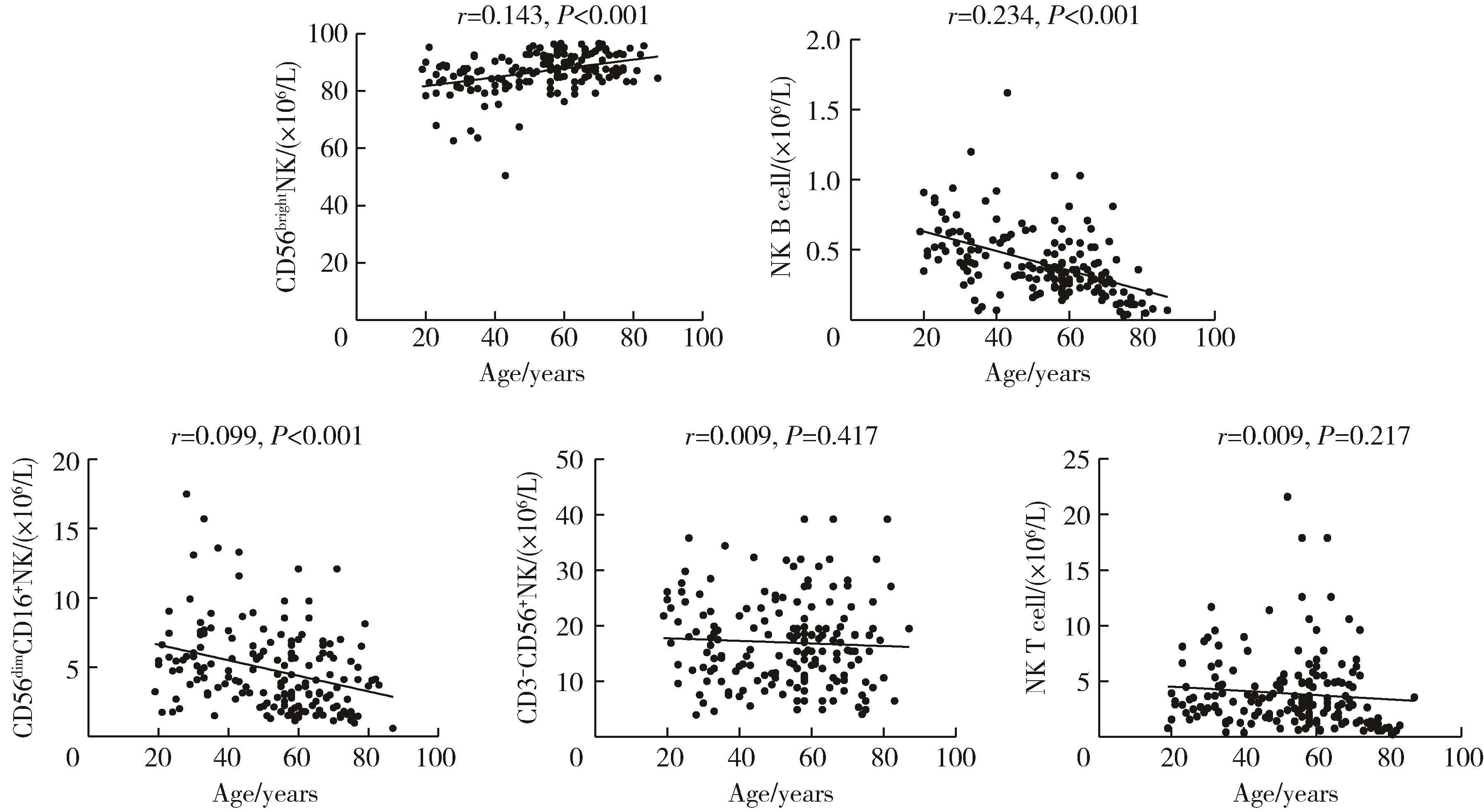
目的: 研究中国健康人外周血中自然杀伤(natural killer,NK)细胞及其亚群的比例和数量,初步确定其正常值范围作为临床检验的参考数值。方法: 选取200例健康成年人,年龄范围为18~87岁,所有受试者被分成6个年龄组:18~30、31~40、41~50、51~60、61~70、71~87岁,收集外周血,以CD16、CD56、CD3、CD19等作为表面标记,使用流式细胞术检测NK细胞及亚群的相对比例及绝对计数。采用SPSS 27.0软件分析数据,计量资料用均数±标准差表示,同时进行t检验、方差分析或秩和检验比较各年龄组和性别组的差异,检验水准α=0.05,以P < 0.05为差异有统计学意义。结果: 200例健康成人受试者CD3-CD56+NK细胞的范围为(13.14±7.56)×106/L,CD56dimCD16+NK细胞的范围为(5.23±3.12)×106/L,CD56brightNK细胞的范围为(85.61±7.40)×106/L,NK T细胞的范围为(4.16±3.34)×106/L,NK B细胞的范围为(0.46±0.24)×106/L。CD3-CD56+NK和NK T细胞在和年龄的相关性方面差异无统计学意义(P=0.417,P=0.217);随着年龄的增长,NK B和CD56dimCD16+NK细胞的数量有一定的下降趋势(r=0.234,P < 0.001;r=0.099,P < 0.001),尤其是在50岁以后更加明显;CD56brightNK则随着年龄增长有上升趋势(r=0.143, P < 0.001)。结论: NK细胞及其亚群的检测对于自身免疫病、感染性疾病和肿瘤的诊断、治疗和预后分析具有重要参考价值;本研究为临床检测NK细胞亚群提供了初步的参考范围,但尚需扩大例数,进行多中心试验以进一步明确。
中图分类号:
- R593.2
| 1 | Yu J , Freud AG , Caligiuri MA . Location and cellular stages of natural killer cell development[J]. Trends Immunol, 2013, 34 (12): 573- 582. |
| 2 | Carrega P , Ferlazzo G . Natural killer cell distribution and trafficking in human tissues[J]. Front Immunol, 2012, 3, 347. |
| 3 | Zhu L , Karakizlis H , Weimer R , et al. Circulating NKG2A-NKG2D+ CD56dimCD16+ natural killer (NK) cells as mediators of functional immunosurveillance in kidney transplant recipients[J]. Ann Transplant, 2020, 25, e925162. |
| 4 | Marras F , Casabianca A , Bozzano F , et al. Control of the HIV-1 DNA reservoir is associated in vivo and in vitro with NKp46/NKp30 (CD335 CD337) inducibility and interferon gamma production by transcriptionally unique NK cells[J]. J Virol, 2017, 91 (23): e00647. |
| 5 | Cichocki F , Grzywacz B , Miller JS . Human NK cell development: One road or many?[J]. Front Immunol, 2019, 10, 2078. |
| 6 | Freud AG , Mundy-Bosse BL , Yu J , et al. The broad spectrum of human natural killer cell diversity[J]. Immunity, 2017, 47 (5): 820- 833. |
| 7 | Melzer S , Zachariae S , Bocsi J , et al. Reference intervals for leukocyte subsets in adults: Results from a population-based study using 10-color flow cytometry[J]. Cytometry B Clin Cytom, 2015, 88 (4): 270- 281. |
| 8 | Shu SA , Wang J , Tao MH , et al. Gene therapy for autoimmune disease[J]. Clin Rev Allergy Immunol, 2015, 49 (2): 163- 176. |
| 9 | Choi J , Lee SJ , Lee YA , et al. Reference values for peripheral blood lymphocyte subsets in a healthy korean population[J]. Immune Netw, 2014, 14 (6): 289- 295. |
| 10 | Yawata N , Selva KJ , Liu YC , et al. Dynamic change in natural killer cell type in the human ocular mucosa in situ as means of immune evasion by adenovirus infection[J]. Mucosal Immunol, 2016, 9 (1): 159- 170. |
| 11 | Tahrali I , Kucuksezer UC , Akdeniz N , et al. CD3-CD56+ NK cells display an inflammatory profile in RR-MS patients[J]. Immunol Lett, 2019, 216, 63- 69. |
| 12 | Bendelac A , Savage PB , Teyton L . The biology of NK T cells[J]. Annu Rev Immunol, 2007, 25, 297- 336. |
| 13 | McCarthy C , Shepherd D , Fleire S , et al. The length of lipids bound to human CD1d molecules modulates the affinity of NK T cell TCR and the threshold of NK T cell activation[J]. J Exp Med, 2007, 204 (5): 1131- 1144. |
| 14 | Simoni Y , Diana J , Ghazarian L , et al. Therapeutic manipulation of natural killer (NK) T cells in autoimmunity: Are we close to reality?[J]. Clin Exp Immunol, 2013, 171 (1): 8- 19. |
| 15 | Li M , Xiong Y , Li M , et al. Depletion but activation of CD56(dim)CD16(+)NK cells in acute infection with severe fever with thrombocytopenia syndrome virus[J]. Virol Sin, 2020, 35 (5): 588- 598. |
| 16 | Gayoso I , Sanchez-Correa B , Campos C , et al. Immunosene-scence of human natural killer cells[J]. J Innate Immun, 2011, 3 (4): 337- 343. |
| 17 | Solana R , Tarazona R , Gayoso I , et al. Innate immunosene-scence: Effect of aging on cells and receptors of the innate immune system in humans[J]. Semin Immunol, 2012, 24 (5): 331- 341. |
| 18 | Solana R , Pawelec G , Tarazona R . Aging and innate immunity[J]. Immunity, 2006, 24 (5): 491- 494. |
| 19 | Cunha CF , Ferraz-Nogueira R , Costa VFA , et al. Contribution of Leishmania braziliensis antigen-specific CD4+ T, CD8+ T, NK and CD3+CD56+NK T cells in the immunopathogenesis of cutaneous leishmaniasis patients: Cytotoxic, activation and exhaustion profiles[J]. PLoS One, 2020, 15 (3): e0229400. |
| 20 | Myers JA , Miller JS . Exploring the NK cell platform for cancer immunotherapy[J]. Nat Rev Clin Oncol, 2021, 18 (2): 85- 100. |
| 21 | Terrén I , Orrantia A , Vitallé J , et al. NK Cell metabolism and tumor microenvironment[J]. Frontiers in immunology, 2019, 10, 2278. |
| 22 | Zhang C , Liu Y . Targeting NK Cell Checkpoint receptors or molecules for cancer immunotherapy[J]. Front Immunol, 2020, 11, 1295. |
| [1] | 赵祥格,刘佳庆,黄会娜,陆智敏,白自然,李霞,祁荆荆. 干扰素-α介导系统性红斑狼疮外周血CD56dimCD57+自然杀伤细胞功能的损伤[J]. 北京大学学报(医学版), 2023, 55(6): 975-981. |
| [2] | 马向波,张学武,贾汝琳,高颖,刘洪江,刘玉芳,李英妮. 外周血淋巴细胞亚群检测在系统性硬化症治疗中的应用[J]. 北京大学学报(医学版), 2021, 53(4): 721-727. |
|
||