北京大学学报(医学版) ›› 2019, Vol. 51 ›› Issue (3): 487-492. doi: 10.19723/j.issn.1671-167X.2019.03.016
荧光分析法测定人体血液样品中脱嘌呤/脱嘧啶核酸内切酶1(APE1)的活性
- 北京分子科学国家研究中心,生物有机与分子工程教育部重点实验室,北京大学化学与分子工程学院,北京 100871
Fluorescence assay for the detection of apurinic/apyrimidinic endonuclease 1 (APE1) activity in human blood samples
- Beijing National Laboratory for Molecular Sciences, MOE Key Laboratory of Bioorganic Chemistry and Molecular Engineering, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China
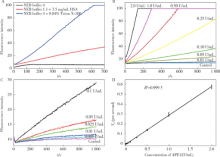
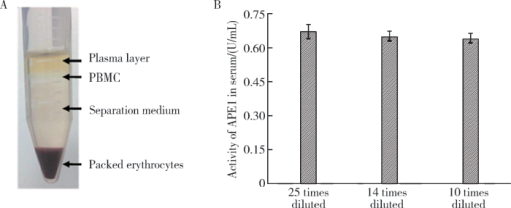
摘要: 目的 开发一种可快速、灵敏地检测生物样品中脱嘌呤/脱嘧啶核酸内切酶1(apurinic/apyrimidinic endonuclease 1,APE1)含量的荧光分析方法。方法 根据APE1所具有的脱碱基核酸内切酶活性,合成了一种用荧光基团与猝灭基团标记的含脱碱基位点的DNA荧光探针。在合适的缓冲体系下,APE1将该DNA探针水解并释放出荧光基团,根据荧光信号上升速率实现对APE1活性的定量检测。在此基础上,本研究改进了检测APE1时溶液缓冲液的条件,使得该荧光探针对APE1的响应更为灵敏,并进一步通过密度梯度离心法从人全血样品提取了外周血单核细胞(peripheral blood mononuclear cells,PBMCs),用改进后的荧光探针法定量测定了其蛋白提取液中APE1的含量。最后,使用该荧光探针法测定了临床血液样本中APE1的含量。结果 本研究建立的方法对APE1的最低检测限和功能灵敏度均为0.005 U/mL(3 pg/mL),线性范围为6 pg/mL~1.2 ng/mL。利用该方法测定了8份人血液样品中PBMCs蛋白中APE1的含量,测得每微克PBMCs蛋白中APE1的含量分布为0.061~0.40 ng,平均含量为0.16 ng APE1,加标回收率为98%±5%(n=3)。用该方法对102份正常人(男51例、女51例,年龄59~75岁)血清样品中的APE1含量进行了检测,得到这些血清样品中APE1含量的分布范围为0.13~0.34 ng/mL,加标回收率为96%±15%(n=3)。结论 本研究发展的荧光分析法操作简便、灵敏度高、所需生物样品量小,能够快速、准确地测定血液等生物样本中的APE1含量,解决了原有方法检测低含量血清样品时误差较大的问题,有良好的临床检验应用前景。
中图分类号:
- R446.1
| [1] |
Li M , Wilson DM 3rd. Human apurinic/apyrimidinic endo-nuclease 1[J]. Antioxid Redox Signal, 2014,20(4):678-707.
doi: 10.1089/ars.2013.5492 |
| [2] |
Whitaker AM, Freudenthal BD . APE1: A skilled nucleic acid surgeon[J]. DNA Repair (Amst), 2018,71:93-100.
doi: 10.1016/j.dnarep.2018.08.012 |
| [3] |
Traversi D, Cervella P, Gilli G . Evaluating the genotoxicity of urban PM2.5 using PCR-based methods in human lung cells and the Salmonella TA98 reverse test[J]. Environ Sci Pollut Res, 2015,22(2):1279-1289.
doi: 10.1007/s11356-014-3435-1 |
| [4] |
Simeonov A, Kulkarni A, Dorjsuren D , et al. Identification and characterization of inhibitors of human apurinic/apyrimidinic endonuclease APE1[J]. PLoS One, 2009,4(6):e5740.
doi: 10.1371/journal.pone.0005740 |
| [5] |
Han J, Zhuo Y, Chai Y , et al. Ultrasensitive electrochemical strategy for trace detection of APE-1 via triple signal amplification strategy[J]. Biosens Bioelectron, 2013,41:116-122.
doi: 10.1016/j.bios.2012.07.082 |
| [6] |
Kreklau EL, Limp-Foster M, Liu N , et al. A novel fluorometric oligonucleotide assay to measure O 6-methylguanine DNA methyltransferase, methylpurine DNA glycosylase, 8-oxoguanine DNA glycosylase and abasic endonuclease activities: DNA repair status in human breast carcinoma cells overexpressing methylpurine DNA glycosylase [J]. Nucleic Acids Res, 2001,29(12):2558-2566.
doi: 10.1093/nar/29.12.2558 |
| [7] | Zhang S, He L, Dai N , et al. Serum APE1 as a predictive marker for platinum-based chemotherapy of non-small cell lung cancer patients[J]. Oncotarget, 2016,7(47):77482-77494. |
| [8] |
Kirkali G, Jaruga P, Reddy P . Identification and quantification of DNA repair protein apurinic/apyrimidinic endonuclease 1 (APE1) in human cells by liquid chromatography/isotope-dilution tandem mass spectrometry[J]. PLoS One, 2013,8(7):e69894.
doi: 10.1371/journal.pone.0069894 |
| [9] |
Coskun E, Jaruga P, Reddy P . Extreme expression of DNA repair protein apurinic/apyrimidinic endonuclease 1 (APE1) in human breast cancer as measured by liquid chromatography and isotope dilution tandem mass spectrometry[J]. Biochemistry, 2015,54(38):5787-5790.
doi: 10.1021/acs.biochem.5b00928 |
| [10] |
Fang S, Chen L, Zhao M . Unimolecular chemically modified DNA fluorescent probe for one-step quantitative measurement of the activity of human apurinic/apyrimidinic endonuclease 1 in biological samples[J]. Anal Chem, 2015,87(24):11952-11956.
doi: 10.1021/acs.analchem.5b03939 |
| [11] |
Greening DW, Simpson RJ . Characterization of the low-molecular-weight human plasma peptidome[J]. Methods Mol Biol, 2017,1619:63-79.
doi: 10.1007/978-1-4939-7057-5 |
| [12] |
Wang D, Zhuo JQ, Zhao MP . A simple and rapid competitive enzyme-linked immunosorbent assay (cELISA) for high-throughput measurement of secretory immunoglobulin A (sIgA) in saliva[J]. Talanta, 2010,82(1):432-436.
doi: 10.1016/j.talanta.2010.04.040 |
| [13] |
Li XJ, Mei X, Huang YF , et al. Simple label-free fluorescence detection of apurinic/apyrimidinic endonuclease 1 activity and its inhibitor using the abasic site-binding fluorophore[J]. Anal Methods, 2019,11(6):739-743.
doi: 10.1039/C8AY02633E |
| [14] |
Evans AR, Limp-Foster M, Kelley MR . Going APE over ref-1[J]. Mutat Res, 2000,461(2):83-108.
doi: 10.1016/S0921-8777(00)00046-X |
| [15] |
Antoniali G, Malfatti MC, Tell G . Unveiling the non-repair face of the base excision repair pathway in RNA processing: A missing link between DNA repair and gene expression?[J]. DNA Repair, 2017,56:65-74.
doi: 10.1016/j.dnarep.2017.06.008 |
| [16] | Kelley MR, Cheng L, Foster R , et al. Elevated and altered expression of the multifunctional DNA base excision repair and redox enzyme Ape1/ref-1 in prostate cancer[J]. Clin Cancer Res, 2001,7(4):824-830. |
| [17] |
Wang D, Xiang DB, Yang XQ , et al. APE1 overexpression is associated with cisplatin resistance in non-small cell lung cancer and targeted inhibition of APE1 enhances the activity of cisplatin in A549 cells[J]. Lung Cancer, 2009,66(3):298-304.
doi: 10.1016/j.lungcan.2009.02.019 |
| [18] | Moore DH, Michael H, Tritt R , et al. Alterations in the expression of the DNA repair/redox enzyme APE/ref-1 in epithelial ovarian cancers[J]. Clin Cancer Res, 2000,6(2):602-609. |
| [19] |
Shin JH, Choi S, Lee YR , et al. APE1/Ref-1 as a serological biomarker for the detection of bladder cancer[J]. Cancer Res Treat, 2015,47(4):823-833.
doi: 10.4143/crt.2014.074 |
| [20] |
Dai N, Cao XJ, Li MX , et al. Serum APE1 autoantibodies: a novel potential tumor marker and predictor of chemotherapeutic efficacy in non-small cell lung cancer[J]. PLoS One, 2013,8(3):e58001.
doi: 10.1371/journal.pone.0058001 |
| [1] | 王鹏,杨子瑶,王萌,王巍,李爱芝. 2例罕见RhD变异型RHD*DEL37的分子生物学分析[J]. 北京大学学报(医学版), 2024, 56(2): 352-356. |
| [2] | 王磊,金香淑,董慧君,欧国敏,赖鑫源,庄辉,李彤,向宽辉. 基于COL1A1启动子和增强型绿色荧光蛋白基因建立人肝星状细胞活化的细胞模型[J]. 北京大学学报(医学版), 2023, 55(5): 876-885. |
| [3] | 吴雨筱,康一帆,毛茜潆,李梓萌,单小峰,蔡志刚. 亚甲蓝近红外荧光在大鼠口腔前哨淋巴结示踪中的应用[J]. 北京大学学报(医学版), 2023, 55(4): 684-688. |
| [4] | 王微,李鑫,柳萍,董颖. 荧光原位杂交检测MDM2和DDIT3基因信号改变在诊断脂肪肉瘤中的价值[J]. 北京大学学报(医学版), 2023, 55(2): 228-233. |
| [5] | 王雪萍,张于亚楠,卢天兰,卢喆,康哲维,孙瑶瑶,岳伟华. 首发精神分裂症肠道微生物多态性与临床症状及血清代谢组学的关联[J]. 北京大学学报(医学版), 2022, 54(5): 863-873. |
| [6] | 杜强,洪锴,潘伯臣. 两种检测男性生殖道沙眼衣原体和解脲支原体方法的对比[J]. 北京大学学报(医学版), 2021, 53(4): 785-788. |
| [7] | 池彦廷,张延平,张秋露,刘翠苓,李斌斌. 唾液腺干燥综合征继发黏膜相关淋巴组织淋巴瘤的临床病理分析[J]. 北京大学学报(医学版), 2021, 53(1): 40-45. |
| [8] | 张树栋,洪鹏,王滨帅,邓绍晖,张帆,陶立元,曹财广,胡振华,马潞林. 吲哚菁绿标记的荧光实时显影技术在腹腔镜肾部分切除术中的应用[J]. 北京大学学报(医学版), 2020, 52(4): 657-662. |
| [9] | 胥佳佳,王燕,孙贺,贾汝琳,张学武,孟洋,任丽丽,孙晓麟. 可溶性白细胞介素2受体α链的检测与类风湿关节炎疾病活动性评估[J]. 北京大学学报(医学版), 2018, 50(6): 975-980. |
| [10] | 吴鹏辉,谢瑶,赵卫红,华瑛,孙青,李硕,吴晔,卢新天. 血液病/肿瘤患儿并发可逆性后部白质脑病综合征[J]. 北京大学学报(医学版), 2018, 50(4): 662-665. |
| [11] | 刘婧寅,陈飞,葛严军,魏菱,潘韶霞,冯海兰. 选择性激光熔化种植体对早期骨矿化沉积率的影响[J]. 北京大学学报(医学版), 2018, 50(1): 117-122. |
| [12] | 张意兰,王智峰,陈宁. 血清IgG4在不同疾病患者中的表达[J]. 北京大学学报(医学版), 2017, 49(6): 961-964. |
| [13] | 冯永良,范静慧,林宪娟,杨吉春,崔庆华,汤新景,徐国恒,耿彬. 荧光探针包被法测定血浆硫化氢浓度[J]. 北京大学学报(医学版), 2017, 49(6): 1060-1065. |
| [14] | 王芳, 张琰琴, 丁洁, 俞礼霞. 多重竞争性荧光PCR检测X连锁Alport综合征大片段缺失突变[J]. 北京大学学报(医学版), 2017, 49(5): 760-767. |
| [15] | 余建峰, 金月波, 何菁, 安媛, 栗占国. 皮肌炎继发干燥综合征伴肺间质病变的血清人Ⅱ型肺泡细胞表面抗原变化1例[J]. 北京大学学报(医学版), 2017, 49(5): 910-914. |
|
||