北京大学学报(医学版) ›› 2020, Vol. 52 ›› Issue (5): 836-844. doi: 10.19723/j.issn.1671-167X.2020.05.007
全外显子组测序和目标序列靶向捕获测序在遗传性视网膜变性基因诊断中的差异
- 北京大学第三医院眼科, 北京 100191
Comparison study of whole exome sequencing and targeted panel sequencing in molecular diagnosis of inherited retinal dystrophies
Xiao-zhen LIU,Ying-ying LI,Li-ping YANG( )
)
- Department of Ophthalmology, Peking University Third Hospital, Beijing 100191, China
摘要:
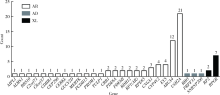
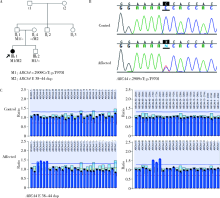
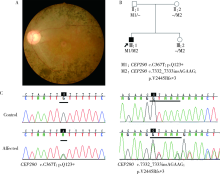
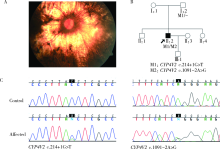
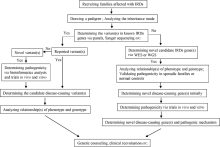
目的:对比外显子组测序(whole exome sequencing,WES)和目标序列靶向捕获测序检测中国遗传性视网膜变性(inherited retinal dystrophies,IRDs)患者致病基因变异的差异。方法:收集182例IRDs家系,所有先证者均接受系统的眼科检查和必要的全身检查,采集患者及家属血样并提取基因组DNA。按照就诊的时间顺序将患者平均分为两组,一组91例接受WES,另一组91例应用本课题组设计并定制的“遗传性眼病基因诊断芯片” (hereditary eye disease enrichment panel,HEDEP)进行IRDs致病基因外显子区域靶向捕获测序。对候选致病基因用Sanger测序进行验证,并对家系成员进行共分离分析,使用多重连接依赖的探针扩增技术对拷贝数变异进行验证,针对二代测序捕获效率低的区域如RPGR ORF15区,应用Sanger 测序补充检测。根据美国医学遗传学与基因组学学会和分子病理学协会(American College of Medical Genetics and Genomics and the Association for Molecular Pathology,ACMG/AMP)制定的《ACMG/AMP基因变异分类标准与指南》将检测到的所有基因变异进行分类,本文只包含“致病的”、“可能致病的”的基因变异,不包含“意义不明确的”、“可能良性的”和“良性的”基因变异。结果:应用HEDEP确诊的家系共51例,阳性率为56.04%(51/91);应用WES确诊的家系共30例,阳性率为33.00%(30/91);总阳性率44.51%(81/182)。平均测序深度以及测序覆盖度方面,HEDEP优于WES,此外HEDEP具有检测拷贝数变异潜力。本研究共检测到29个IRDs基因的致病突变,最常见的致病基因为USH2A、ABCA4和RPGR,基因突变频率分别为11.54%(21/182)、6.59%(12/182)、3.85%(7/182);共发现43个新的致病突变,并检测到6例家系携带RPGR ORF15区的突变。结论:针对临床确诊的IRDs病例,HEDEP较WES能获得更高的基因诊断阳性率和更精确的诊断结果,可作为IRDs基因诊断的首选方法,WES可作为其他基因诊断方法的补充手段。同时,本研究丰富了IRDs致病基因的突变频谱,为将来IRDs基因诊断、遗传咨询和基因治疗奠定了基础。
中图分类号:
- R774.13
| [1] |
Berger W, Kloeckener-Gruissem B, Neidhardt J. The molecular basis of human retinal and vitreoretinal diseases[J]. Prog Retin Eye Res, 2010,29(5):335-375.
doi: 10.1016/j.preteyeres.2010.03.004 |
| [2] |
Zhao L, Wang F, Wang H, et al. Next-generation sequencing-based molecular diagnosis of 82 retinitis pigmentosa probands from Northern Ireland[J]. Hum Genet, 2015,134(2):217-230.
doi: 10.1007/s00439-014-1512-7 pmid: 25472526 |
| [3] |
Glockle N, Konl LS, Mohr J, et al. Panel-based next generation sequencing as a reliable and efficient technique to detect mutations in unselected patients with retinal dystrophies[J]. Eur J Hum Genet, 2014,22(1):99-104.
doi: 10.1038/ejhg.2013.72 |
| [4] | Beryozkin A, Shevah E, Kimchi A, et al. Whole exome sequencing reveals mutations in known retinal disease genes in 33 out of 68 Israeli families with inherited retinopathies[J]. Sci Rep, 2015(5):13187. |
| [5] |
Yang L, Cui H, Yin X, et al. Dependable and efficient clinical molecular diagnosis of Chinese RP patient with targeted exon sequencing[J]. PLoS One, 2015,10(10):e0140684.
doi: 10.1371/journal.pone.0140684 pmid: 26496393 |
| [6] | Riera M, Navarro R, Ruiz-Nogales S, et al. Whole exome sequencing using Ion Proton system enables reliable genetic diagnosis of inherited retinal dystrophies[J]. Sci Rep, 2017(7):42078. |
| [7] |
Zhang J, Wang C, Shen Y, et al. A mutation in ADIPOR1 causes nonsyndromic autosomal dominant retinitis pigmentosa[J]. Hum Genet, 2016,135(12):1375-1387.
doi: 10.1007/s00439-016-1730-2 pmid: 27655171 |
| [8] |
Bader I, Brandau O, Achatz H, et al. X-linked retinitis pigmentosa: RPGR mutations in most families with definite X linkage and clustering of mutations in a short sequence stretch of exon ORF15[J]. Invest Ophthalmol Vis Sci, 2003,44(4):1458-1463.
doi: 10.1167/iovs.02-0605 pmid: 12657579 |
| [9] |
Li L, Xiao X, Li S, et al. Detection of variants in 15 genes in 87 unrelated Chinese patients with Leber congenital amaurosis[J]. PLoS One, 2011,6(5):e19458.
doi: 10.1371/journal.pone.0019458 pmid: 21602930 |
| [10] |
Xiao X, Mai G, Li S, et al. Identification of CYP4V2 mutation in 21 families and overview of mutation spectrum in Bietti crystalline corneoretinal dystrophy[J]. Biochem Biophys Res Commun, 2011,409(2):181-186.
doi: 10.1016/j.bbrc.2011.04.112 pmid: 21565171 |
| [11] |
Li A, Jiao X, Munier FL, et al. Bietti crystalline corneoretinal dystrophy is caused by mutations in the novel gene CYP4V2[J]. Am J Hum Genet, 2004,74(5):817-826.
doi: 10.1086/383228 |
| [12] |
Consugar MB, Navarro-Gomez D, Place EM, et al. Panel-based genetic diagnostic testing for inherited eye diseases is highly accurate and reproducible, and more sensitive for variant detection, than exome sequencing[J]. Genet Med, 2015,17(4):253-261.
doi: 10.1038/gim.2014.172 pmid: 25412400 |
| [13] |
Bujakowska KM, Fernandez-Godino R, Place E, et al. Copy-number variation is an important contributor to the genetic causality of inherited retinal degenerations[J]. Genet Med, 2017,19(6):643-651.
doi: 10.1038/gim.2016.158 pmid: 27735924 |
| [14] |
Haer-Wigman L, van Zelst-Stams WA, Pfundt R, et al. Diagnostic exome sequencing in 266 Dutch patients with visual impairment[J]. Eur J Hum Genet, 2017,25(5):591-599.
doi: 10.1038/ejhg.2017.9 pmid: 28224992 |
| [15] | Broadgate S, Yu J, Downes SM, et al. Unravelling the genetics of inherited retinal dystrophies: Past, present and future[J]. Prog Retin Eye Res, 2017(59):53-96. |
| [16] | 周雪莹, 于志强. 全基因组外显子测序在眼科遗传病中的应用[J]. 中华眼科杂志, 2015,51(5):395-400. |
| [17] |
ACMG Board of Directors Points to consider in the clinical application of genomic sequencing[J]. Genet Med, 2012,14(8):759-761.
doi: 10.1038/gim.2012.74 pmid: 22863877 |
| [18] |
Yuzawa M, Mae Y, Matsui M. Bietti’s crystalline retinopathy[J]. Ophthalmic Paediatr Genet, 1986,7(1):9-20.
doi: 10.3109/13816818609058037 pmid: 3703493 |
| [19] |
Lee KY, Koh AH, Aung T, et al. Characterization of Bietti crystalline dystrophy patients with CYP4V2 mutations[J]. Invest Ophthalmol Vis Sci, 2005,46(10):3812-3816.
pmid: 16186368 |
| [20] |
Tiwari A, Lemke J, Altmueller J, et al. Identification of novel and recurrent disease-causing mutations in retinal dystrophies using whole exome sequencing (WES): Benefits and limitations[J]. PLoS One, 2016,11(7):e0158692.
doi: 10.1371/journal.pone.0158692 pmid: 27391102 |
| [1] | 谢尚,蔡志刚,单小峰. 全外显子测序及相关指标在口腔鳞状细胞癌精准治疗中的应用价值[J]. 北京大学学报(医学版), 2023, 55(4): 697-701. |
| [2] | 冯科,倪菁菁,夏彦清,曲晓伟,张慧娟,万锋,洪锴,张翠莲,郭海彬. 3例SUN5基因变异导致无头精子症的遗传学分析和助孕治疗结局[J]. 北京大学学报(医学版), 2021, 53(4): 803-807. |
| [3] | 李芳,刘洋,刘浩辰,冯海兰. 乳光牙本质患者的基因变异分析及患牙的组织学观察[J]. 北京大学学报(医学版), 2018, 50(4): 666-671. |
| [4] | 吴恺,张杨,张红,檀增桓,郭晓蕙,杨建梅. 嗜铬细胞瘤/副神经节瘤患者RET、VHL、SDHD、SDHB遗传基因变异的检测[J]. 北京大学学报(医学版), 2018, 50(4): 634-639. |
|
||