北京大学学报(医学版) ›› 2021, Vol. 53 ›› Issue (3): 439-446. doi: 10.19723/j.issn.1671-167X.2021.03.001
• 论著 • 下一篇
长度和化学修饰在多壁碳纳米管诱导内皮细胞活化中的作用
- 北京大学公共卫生学院劳动卫生与环境卫生学系,北京 100191
Effects of length and chemical modification on the activation of vascular endothelial cells induced by multi-walled carbon nanotubes
SHEN Jie,YANG Di,CHEN Meng-yuan,GUO Xin-biaoΔ( )
)
- Department of Occupational and Environmental Health, Peking University School of Public Health, Beijing 100191, China
摘要:
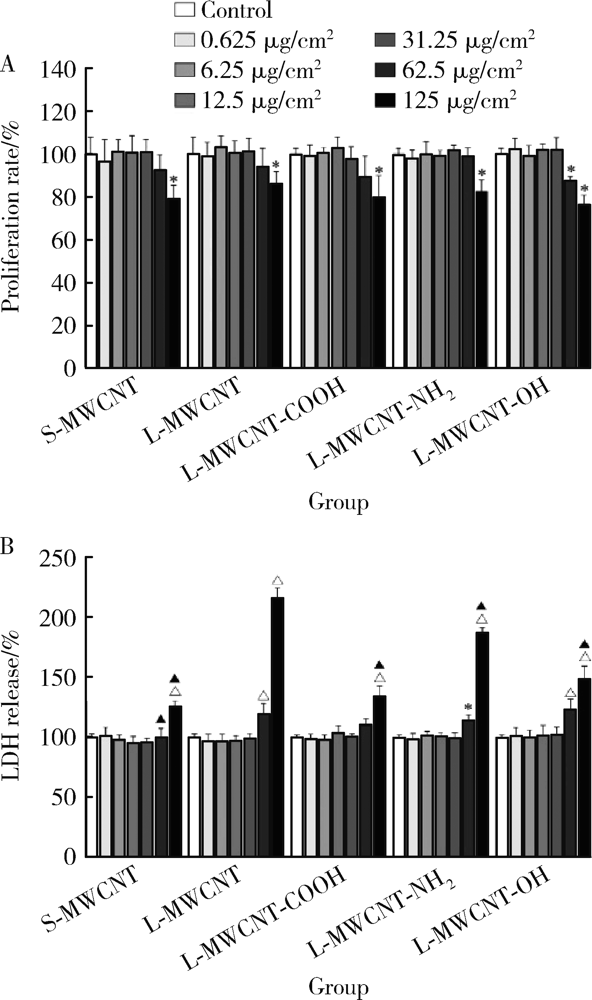
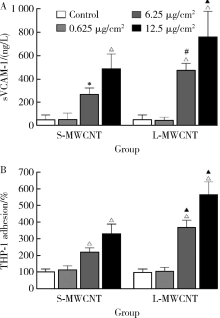
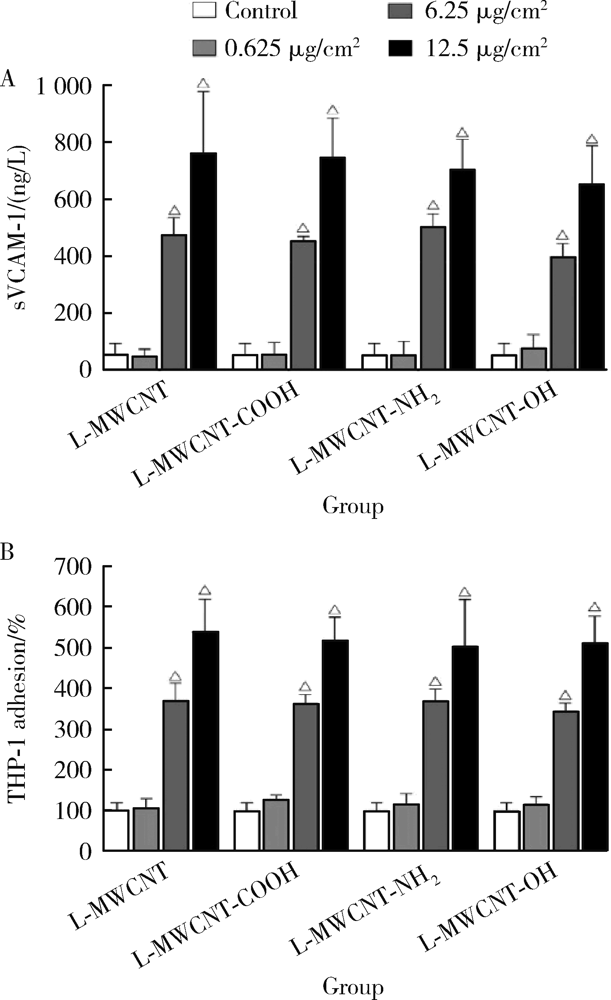
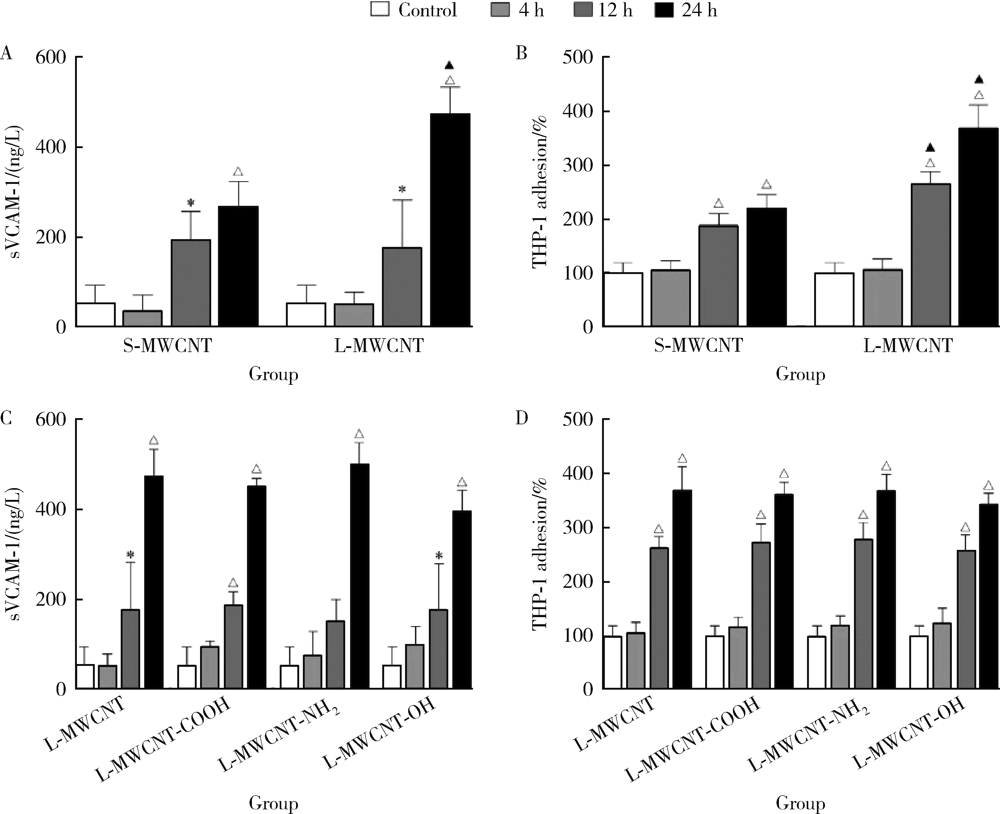
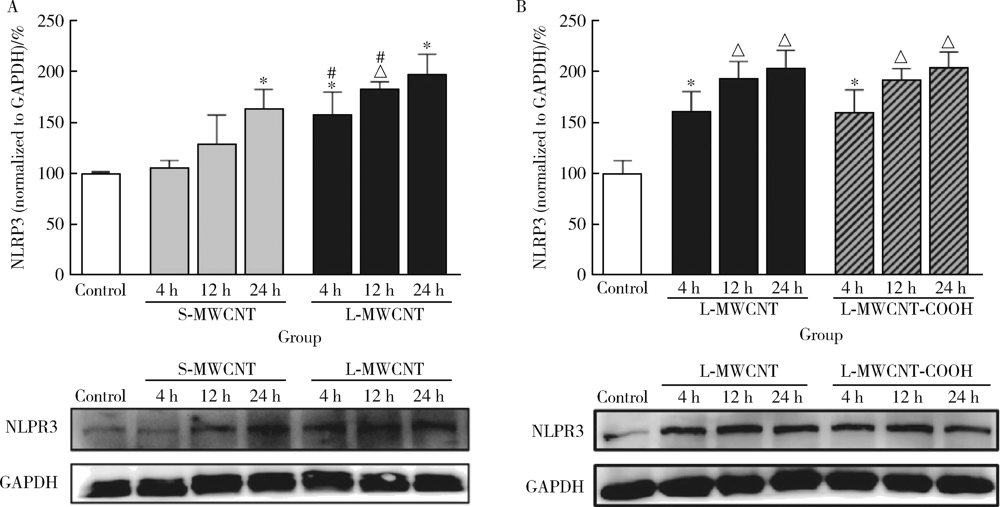
目的: 研究比较不同长度和化学修饰的多壁碳纳米管(multi-walled carbon nanotubes,MWCNTs)对内皮细胞的活化作用,并探讨核苷酸结合寡聚结构域样受体家族含pyrin结构域蛋白3(nucleotide-binding oligomerization domain-like receptor family pyrin domain containing 3,NLRP3)炎性小体的相关机制。方法: 采用动态光散射法对MWCNTs悬液进行表征,将不同长度[短MWCNT(short MWCNT, S-MWCNT) 0.5~2.0 μm或长MWCNT(long MWCNT, L-MWCNT) 10~30 μm]或相同长度(10~30 μm)下不同化学修饰的MWCNTs[未修饰(L-MWCNT)、羧基修饰(L-MWCNT-COOH)、氨基修饰(L-MWCNT-NH2)和羟基修饰(L-MWCNT-OH)]作用于小鼠脑微血管内皮细胞系b.End3。分别采用细胞增殖检测(cell counting kit,CCK)-8试验和乳酸脱氢酶释放试验评价MWCNTs的细胞毒性,通过酶联免疫吸附试验测定胞外血管细胞黏附分子-1(vascular cell adhesion molecule-1,VCAM-1)含量并进一步使用人单核细胞系THP-1进行黏附力试验,评价不同种类MWCNTs的内皮细胞活化效应。进一步选择S-MWCNT、L-MWCNT和L-MWCNT-COOH探讨内皮细胞活化的炎症机制,采用免疫蛋白印迹法检测MWCNTs作用后细胞炎性小体蛋白NLRP3的表达水平。结果: 在较高浓度(125 μg/cm2)下作用24 h,不同种类的MWCNTs均可显著抑制b.End3的细胞活性并影响细胞膜完整性。在无明显细胞毒性的浓度下(6.25 μg/cm2),不同种类的MWCNTs作用12 h均可显著诱导b.End3细胞内皮细胞活化,表现为VCAM-1释放水平增加,以及b.End3对THP-1的黏附水平增强。相同浓度下,L-MWCNT诱导细胞内皮细胞活化的效应显著强于S-MWCNT;相同长度下,L-MWCNT和L-MWCNT-COOH对细胞内皮细胞活化的效应差异无统计学意义。在6.25 μg/cm2浓度下,S-MWCNT、L-MWCNT、L-MWCNT-COOH可时间依赖性地上调b.End3细胞的NLRP3蛋白表达水平。与相同浓度的S-MWCNT相比,L-MWCNT可显著上调细胞NLRP3的蛋白表达水平;在相同长度下,L-MWCNT和L-MWCNT-COOH作用后细胞的NLRP3蛋白表达差异无统计学意义。结论: 与化学修饰作用相比,MWCNTs长度增加更易诱导内皮细胞活化,其中间机制可能与NLRP3炎性小体的激活有关。MWCNTs的安全性评价应关注其理化特性对血管内皮生物效应的影响。
中图分类号:
- R12
| [1] | He H, Pham-Huy LA, Dramou P, et al. Carbon nanotubes: applications in pharmacy and medicine[J]. Biomed Res Int, 2013,2013:578290. |
| [2] |
Aschberger K, Johnston HJ, Stone V, et al. Review of carbon nanotubes toxicity and exposure: appraisal of human health risk assessment based on open literature[J]. Crit Rev Toxicol, 2010,40(9):759-790.
doi: 10.3109/10408444.2010.506638 |
| [3] |
Amenta V, Aschberger K. Carbon nanotubes: potential medical applications and safety concerns[J]. Wiley Interdiscip Rev Nanomed Nanobiotechnol, 2015,7(3):371-386.
doi: 10.1002/wnan.2015.7.issue-3 |
| [4] | John AA, Subramanian AP, Vellayappan MV, et al. Carbon nanotubes and graphene as emerging candidates in neuroregeneration and neurodrug delivery[J]. Int J Nanomedicine, 2015,10:4267-4277. |
| [5] |
Gonzalez-Carter D, Goode AE, Kiryushko D, et al. Quantification of blood-brain barrier transport and neuronal toxicity of unlabelled multiwalled carbon nanotubes as a function of surface charge[J]. Nanoscale, 2019,11(45):22054-22069.
doi: 10.1039/c9nr02866h pmid: 31720664 |
| [6] |
Setyawati MI, Tay CY, Docter D, et al. Understanding and exploiting nanoparticles’ intimacy with the blood vessel and blood[J]. Chem Soc Rev, 2015,44(22):8174-8199.
doi: 10.1039/c5cs00499c pmid: 26239875 |
| [7] |
Hunt BJ, Jurd KM. Endothelial cell activation. A central pathophysiological process[J]. BMJ, 1998,316(7141):1328-1329.
pmid: 9563977 |
| [8] |
Zhang J, Defelice AF, Hanig JP, et al. Biomarkers of endothelial cell activation serve as potential surrogate markers for drug-induced vascular injury[J]. Toxicol Pathol, 2010,38(6):856-871.
doi: 10.1177/0192623310378866 pmid: 20716788 |
| [9] |
Schroder K, Tschopp J. The inflammasomes[J]. Cell, 2010,140(6):821-832.
doi: 10.1016/j.cell.2010.01.040 pmid: 20303873 |
| [10] |
van der Heijden T, Kritikou E, Venema W, et al. NLRP3 inflammasome inhibition by MCC950 reduces atherosclerotic lesion development in apolipoprotein E-deficient mice-brief report[J]. Arterioscler Thromb Vasc Biol, 2017,37(8):1457-1461.
doi: 10.1161/ATVBAHA.117.309575 |
| [11] |
Yang WL, Sharma A, Wang Z, et al. Cold-inducible RNA-binding protein causes endothelial dysfunction via activation of NLRP3 inflammasome[J]. Sci Rep, 2016,6:26571.
doi: 10.1038/srep26571 |
| [12] |
Yang D, Shen J, Fan J, et al. Paracellular permeability changes induced by multi-walled carbon nanotubes in brain endothelial cells and associated roles of hemichannels[J]. Toxicology, 2020,440:152491.
doi: S0300-483X(20)30130-X pmid: 32413421 |
| [13] |
Fan J, Chen Y, Yang D, et al. Multi-walled carbon nanotubes induce IL-1β secretion by activating hemichannels-mediated ATP release in THP-1 macrophages[J]. Nanotoxicology, 2020,14(7):929-946.
doi: 10.1080/17435390.2020.1777476 |
| [14] |
Bicker J, Alves G, Fortuna A, et al. Blood-brain barrier models and their relevance for a successful development of CNS drug delivery systems: a review[J]. Eur J Pharm Biopharm, 2014,87(3):409-432.
doi: 10.1016/j.ejpb.2014.03.012 pmid: 24686194 |
| [15] |
Constantinescu CA, Fuior EV, Rebleanu D, et al. Targeted transfection using PEGylated cationic liposomes directed towards P-selectin increases siRNA delivery into activated endothelial cells[J]. Pharmaceutics, 2019,11(1):47.
doi: 10.3390/pharmaceutics11010047 |
| [16] |
Li Y, Cao J. The impact of multi-walled carbon nanotubes (MWCNTs) on macrophages: contribution of MWCNT characteristics[J]. Sci China Life Sci, 2018,61(11):1333-1351.
doi: 10.1007/s11427-017-9242-3 |
| [17] | 龙继敏. 多壁碳纳米管的长度及功能化对血管健康效应的影响及其作用机制[D]. 湖南湘潭: 湘潭大学, 2019. |
| [18] |
Mallakpour S, Khodadadzadeh L. Ultrasonic-assisted fabrication of starch/MWCNT-glucose nanocomposites for drug delivery[J]. Ultrason Sonochem, 2018,40(Pt A):402-409.
doi: S1350-4177(17)30336-X pmid: 28946439 |
| [19] |
Wang JT, Rubio N, Kafa H, et al. Kinetics of functionalised carbon nanotube distribution in mouse brain after systemic injection: Spatial to ultra-structural analyses[J]. J Control Release, 2016,224:22-32.
doi: 10.1016/j.jconrel.2015.12.039 |
| [20] |
Badea MA, Prodana M, Dinischiotu A, et al. Cisplatin loaded multiwalled carbon nanotubes induce resistance in triple negative breast cancer cells[J]. Pharmaceutics, 2018,10(4):228.
doi: 10.3390/pharmaceutics10040228 |
| [21] |
Chen H, Shi Y, Sun L, et al. Electrospun composite nanofibers with all-trans retinoic acid and MWCNTs-OH against cancer stem cells[J]. Life Sci, 2020,258:118152.
doi: 10.1016/j.lfs.2020.118152 |
| [22] |
Kafa H, Wang JT, Rubio N, et al. The interaction of carbon nanotubes with an in vitro blood-brain barrier model and mouse brain in vivo[J]. Biomaterials, 2015,53:437-452.
doi: 10.1016/j.biomaterials.2015.02.083 |
| [23] |
Cao Y, Jacobsen NR, Danielsen PH, et al. Vascular effects of multiwalled carbon nanotubes in dyslipidemic ApoE-/- mice and cultured endothelial cells[J]. Toxicol Sci, 2014,138(1):104-116.
doi: 10.1093/toxsci/kft328 |
| [24] |
Long J, Li X, Kang Y, et al. Internalization, cytotoxicity, oxidative stress and inflammation of multi-walled carbon nanotubes in human endothelial cells: influence of pre-incubation with bovine serum albumin[J]. RSC Advances, 2018,8(17):9253-9260.
doi: 10.1039/C8RA00445E |
| [25] |
Li Z, Liu T, Long J, et al. The toxicity of hydroxylated and carboxylated multi-walled carbon nanotubes to human endothelial cells was not exacerbated by ER stress inducer[J]. Chin Chem Lett, 2019,30(3):582-586.
doi: 10.1016/j.cclet.2018.12.011 |
| [26] |
Svadlakova T, Hubatka F, Turanek Knotigova P, et al. Proinflammatory effect of carbon-based nanomaterials: in vitro study on stimulation of inflammasome NLRP3 via destabilisation of lysosomes[J]. Nanomaterials (Basel), 2020,10(3):418.
doi: 10.3390/nano10030418 |
| [27] |
Sun B, Wang X, Ji Z, et al. NADPH oxidase-dependent NLRP3 inflammasome activation and its important role in lung fibrosis by multiwalled carbon nanotubes[J]. Small, 2015,11(17):2087-2097.
doi: 10.1002/smll.v11.17 |
| [1] | 张帆,陈曲,郝一昌,颜野,刘承,黄毅,马潞林. 术前及术后膜性尿道长度与腹腔镜根治性前列腺切除术后控尿功能恢复的相关性[J]. 北京大学学报(医学版), 2022, 54(2): 299-303. |
| [2] | 田雨, 徐莉, 孟焕新, 任秀云, 陈智滨, 张立, 刘凯宁. 侵袭性牙周炎维生素D受体基因多态性的FBAT分析[J]. 北京大学学报(医学版), 2010, 42(1): 28-32. |
| [3] | 邓芙蓉, 郭新彪, 陈丽, 王志全, 张凯. 5,10-亚甲基四氢叶酸还酶基因多态性与砷甲基化代谢的关系[J]. 北京大学学报(医学版), 2007, 39(2): 149-152. |
| [4] | 裴丽君, 李智文, 张卫, 任爱国, 朱慧萍, 郝玲, 朱江辉, 李竹. 神经管畸形与还原叶酸载体基因(RFC1 A80G)多态性及可疑危险因素的流行病学研究[J]. 北京大学学报(医学版), 2005, 37(4): 341-345. |
| [5] | 朱锦明, 杨孜, 余梅, 王荣, 叶蓉华, 杨惠霞, 翟桂荣, 王琪. 北京1 200例汉族人群中长链脂肪酸氧化酶G1528C基因突变筛查[J]. 北京大学学报(医学版), 2005, 37(1): 72-74. |
| [6] | 蔡林, 张建中. 用PCR-RFLP技术检测皮肤感染性肉芽肿组织中的分支杆菌[J]. 北京大学学报(医学版), 2004, 36(5): 462-465. |
|
||