北京大学学报(医学版) ›› 2021, Vol. 53 ›› Issue (6): 1055-1060. doi: 10.19723/j.issn.1671-167X.2021.06.008
血清YKL-40在诊断抗黑色素瘤分化相关基因5阳性皮肌炎合并严重肺损伤中的价值
张朴丽1,2,杨红霞1,2,张立宁1,2,葛勇鹏1,彭清林1,王国春1,卢昕1,△( )
)
- 1.中日友好医院风湿免疫科
2.北京大学中日友好临床医学院,北京 100029
Value of serum YKL-40 in the diagnosis of anti-MDA5-positive patients with dermatomyositis complicated with severe pulmonary injury
ZHANG Pu-li1,2,YANG Hong-xia1,2,ZHANG Li-ning1,2,GE Yong-peng1,PENG Qing-lin1,WANG Guo-chun1,LU Xin1,△( )
)
- 1. Department of Rheumatology, China-Japan Friendship Hospital, Beijing 100029, China
2. Peking University China-Japan Friendship School of Clinical Medicine, Beijing 100029, China
摘要:
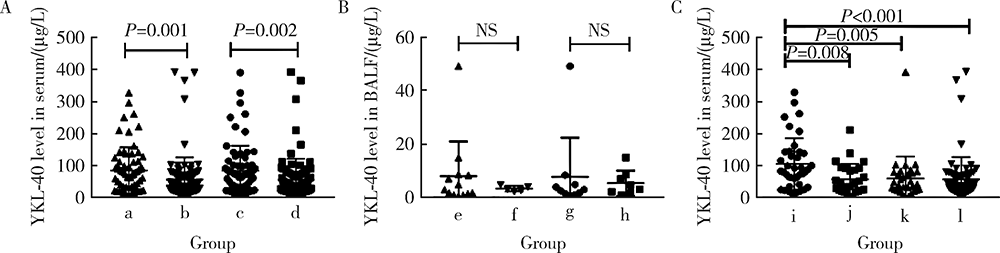
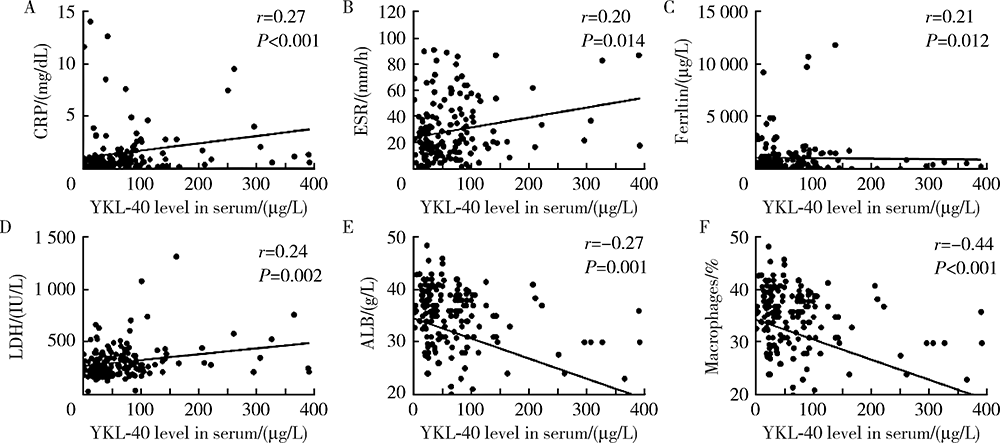
目的:研究血清及支气管肺泡灌洗液(bronchoalveolar lavage fluid, BALF)中YKL-40(chitinase-3-like-1 protein)在抗黑色素瘤分化相关基因5(anti-melanoma differentiation-associated gene 5, MDA5)阳性皮肌炎(dermatomyositis, DM)合并严重肺损伤中的价值,严重肺损伤包括快速进展间质性肺病(rapidly progressive interstitial lung disease, RP-ILD)和肺部感染。方法:选择2013—2018年中日友好医院风湿免疫科住院的抗MDA5阳性DM患者的病例资料进行回顾性分析,收集患者的人口学信息,临床、实验室及影像学检查资料,应用酶联免疫吸附法检测患者血清和BALF中YKL-40水平。绘制受试者工作特征(receiver operating characteristic, ROC)曲线,计算曲线下面积(area under the curve, AUC),评估血清YKL-40对肺损伤的诊断效能。间质性肺病(interstitial lung disease, ILD)由胸部高分辨率CT(high-resolution CT, HRCT)证实。RP-ILD定义为呼吸道症状在3个月内进行性加重,出现呼吸困难和低氧血症,或胸部HRCT显示ILD较之前加重或出现新的ILD。肺部感染经痰、血液、BALF、肺穿刺活检样本检验出病原体确诊。结果:共收集到168例抗MDA5阳性DM患者病例,其中154例合并ILD,66例(39.3%)表现为RP-ILD。经病原学依据证实合并肺部感染患者70例。合并RP-ILD患者中39例(59.1%)合并肺部感染,而非RP-ILD患者仅31例(30.4%)合并肺部感染。RP-ILD合并肺部感染的发生率高于非RP-ILD合并肺部感染者(P<0.001)。血清YKL-40水平在RP-ILD合并肺部感染组高于RP-ILD未合并肺部感染组、非RP-ILD合并肺部感染组和非RP-ILD未合并肺部感染组[83(42~142) vs. 42(21~91) vs. 43(24~79) vs. 38(22~69), P<0.01]。血清YKL-40诊断抗MDA5阳性DM患者RP-ILD合并肺部感染的敏感性、特异性及AUC分别为75%、67%、0.72,其诊断同时存在RP-ILD和肺部感染的抗MDA5阳性DM患者的AUC较诊断仅有RP-ILD和仅有肺部感染者的AUC高,且差异有统计学意义(0.72 vs. 0.54和0.55, Z=2.10和2.11, P<0.05)。结论:抗MDA5阳性DM患者合并RP-ILD和肺部感染预后差,血清YKL-40水平对这类患者同时合并RP-ILD和肺部感染有一定的诊断价值。
中图分类号:
- R593.26
| [1] |
Gupta R, Kumar S, Gow P, et al. Anti-MDA5-associated dermatomyositis[J]. Intern Med J, 2020, 50(4):484-487.
doi: 10.1111/imj.v50.4 |
| [2] |
Lian X, Zou J, Guo Q, et al. Mortality risk prediction in amyopathic dermatomysitis associated with interstitial lung disease: The FLAIR model[J]. Chest, 2020, 158(4):1535-1545.
doi: 10.1016/j.chest.2020.04.057 |
| [3] | Ge YP, Shu XM, He LR, et al. Infection is not rare in patients with idiopathic inflammatory myopathies[J/OL]. Clin Exp Rheumatol, 2021(2021-07-21)[2021-08-01]. https://pubmed.ncbi.nlm.nih.gov/34369354/. |
| [4] |
Wu C, Wang Q, He L, et al. Hospitalization mortality and associated risk factors in patients with polymyositis and dermatomyositis: A retrospective case-control study[J]. PLoS One, 2018, 13(2):e0192491.
doi: 10.1371/journal.pone.0192491 |
| [5] | Yeo IJ, Lee CK, Han SB, et al. Roles of chitinase 3-like 1 in the development of cancer, neurodegenerative diseases, and inflammatory diseases[J/OL]. Pharmacol Ther, 2019, 203(2019-07-26)[2021-08-01]. https://doi.org/10.1016/j.pharmthera.2019.107394. |
| [6] |
Furuhashi K, Suda T, Nakamura Y, et al. Increased expression of YKL-40, a chitinase-like protein, in serum and lung of patients with idiopathic pulmonary fibrosis[J]. Respir Med, 2010, 104(8):1204-1210.
doi: 10.1016/j.rmed.2010.02.026 pmid: 20347285 |
| [7] |
Kornblit B, Hellemann D, Munthe-Fog L, et al. Plasma YKL-40 and CHI3L1 in systemic inflammation and sepsis-experience from two prospective cohorts[J]. Immunobiology, 2013, 218(10):1227-1234.
doi: 10.1016/j.imbio.2013.04.010 pmid: 23706599 |
| [8] |
Spoorenberg SM, Vestjens SM, Rijkers GT, et al. YKL-40, CCL18 and SP-D predict mortality in patients hospitalized with community-acquired pneumonia[J]. Respirology, 2017, 22(3):542-550.
doi: 10.1111/resp.12924 pmid: 27782361 |
| [9] | Hozumi H, Fujisawa T, Enomoto N, et al. Clinical utility of YKL-40 in polymyositis/dermatomyositis-associated interstitial lung disease[J]. J Rheumatol, 2017, 44(9):1394-1401. |
| [10] |
Jiang L, Wang Y, Peng Q, et al. Serum YKL-40 level is associated with severity of interstitial lung disease and poor prognosis in dermatomyositis with anti-MDA5 antibody[J]. Clin Rheumatol, 2019, 38(6):1655-1663.
doi: 10.1007/s10067-019-04457-w pmid: 30739212 |
| [11] |
Lundberg IE, Tjärnlund A, Bottai M, et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups[J]. Ann Rheum Dis, 2017, 76(12):1955-1964.
doi: 10.1136/annrheumdis-2017-211468 pmid: 29079590 |
| [12] |
Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias[J]. Am J Respir Crit Care Med, 2013, 188(6):733-748.
doi: 10.1164/rccm.201308-1483ST |
| [13] |
Moghadam-Kia S, Oddis CV, Aggarwal R. Anti-MDA5 antibody spectrum in western world[J]. Curr Rheumatol Rep, 2018, 20(12):78.
doi: 10.1007/s11926-018-0798-1 pmid: 30382445 |
| [14] |
Sugiyama Y, Yoshimi R, Tamura M, et al. The predictive prognostic factors for polymyositis/dermatomyositis-associated interstitial lung disease[J]. Arthritis Res Ther, 2018, 20(1):7.
doi: 10.1186/s13075-017-1506-7 pmid: 29325580 |
| [15] |
James AJ, Reinius LE, Verhoek M, et al. Increased YKL-40 and chitotriosidase in asthma and chronic obstructive pulmonary disease[J]. Am J Respir Crit Care Med, 2016, 193(2):131-142.
doi: 10.1164/rccm.201504-0760OC |
| [16] |
Korthagen NM, van Moorsel CH, Barlo NP, et al. Serum and BALF YKL-40 levels are predictors of survival in idiopathic pulmonary fibrosis[J]. Respir Med, 2011, 105(1):106-113.
doi: 10.1016/j.rmed.2010.09.012 pmid: 20888745 |
| [17] | Fantino E, Gangell CL, Hartl D, et al. Airway, but not serum or urinary, levels of YKL-40 reflect inflammation in early cystic fibrosis lung disease[J/OL]. BMC Pulm Med, 2014, 14: 28[2021-08-01]. https://doi.org/10.1186/1471-2466-14-28. |
| [18] |
Wang HL, Hsiao PC, Tsai HT, et al. Usefulness of plasma YKL-40 in management of community-acquired pneumonia severity in patients[J]. Int J Mol Sci, 2013, 14(11):22817-22825.
doi: 10.3390/ijms141122817 |
| [19] |
Yang X, Sheng G. YKL-40 levels are associated with disease severity and prognosis of viral pneumonia, but not available in bacterial pneumonia in children[J]. BMC Pediatr, 2018, 18(1):381.
doi: 10.1186/s12887-018-1345-y |
| [20] | Long X, Xuan H, Ohshimo S, et al. Serum YKL-40 as predictor of outcome in hypersensitivity pneumonitis[J/OL]. Eur Respir J, 2016, 49(2): 1501924 [2021-08-01]. https://doi.org/10.1183/13993003.01924-2015. |
| [21] |
Shirakashi M, Nakashima R, Tsuji H, et al. Efficacy of plasma exchange in anti-MDA5-positive dermatomyositis with interstitial lung disease under combined immunosuppressive treatment[J]. Rheumatology (Oxford), 2020, 59(11):3284-3292.
doi: 10.1093/rheumatology/keaa123 |
| [1] | 邢晓燕,张筠肖,朱冯赟智,王一帆,周新尧,李玉慧. 皮肌炎合并巨噬细胞活化综合征5例[J]. 北京大学学报(医学版), 2022, 54(6): 1214-1218. |
| [2] | 吴燕芳,高飞,林滇恬,陈志涵,林禾. 托法替布联合治疗抗MDA5抗体阳性的无肌病皮肌炎并发快速进展型间质性肺病5例临床分析[J]. 北京大学学报(医学版), 2021, 53(5): 1012-1016. |
| [3] | 甘雨舟,李玉慧,张丽华,马琳,何文雯,金月波,安媛,栗占国,叶华. 临床无肌病性皮肌炎与皮肌炎临床及免疫学特征比较[J]. 北京大学学报(医学版), 2020, 52(6): 1001-1008. |
| [4] | 徐婧,徐静,李鹤,唐杰,舒建龙,张婧,石连杰,李胜光. 皮肌炎合并IgA血管炎1例[J]. 北京大学学报(医学版), 2019, 51(6): 1173-1177. |
| [5] | 杨伊莹,左晓霞,朱红林,刘思佳. 皮肌炎/多肌炎表观遗传学标志物的研究进展[J]. 北京大学学报(医学版), 2019, 51(2): 374-377. |
| [6] | 余建峰, 金月波, 何菁, 安媛, 栗占国. 皮肌炎继发干燥综合征伴肺间质病变的血清人Ⅱ型肺泡细胞表面抗原变化1例[J]. 北京大学学报(医学版), 2017, 49(5): 910-914. |
| [7] | 刘爽, 安媛, 贾园, 栗占国. 类风湿关节炎合并无肌病性皮肌炎伴多重肺损伤1例[J]. 北京大学学报(医学版), 2014, 46(5): 805-808. |
| [8] | 陈芳, 舒晓明, 王冬雪, 王国春, 卢昕. 多发性肌炎及皮肌炎患者血清单核细胞趋化蛋白-1的测定及临床意义[J]. 北京大学学报(医学版), 2012, 44(2): 204-208. |
| [9] | 姚海红, 李玉慧, 张学武, 栗占国. 皮肌炎合并甲状腺功能异常的临床及免疫学特征分析[J]. 北京大学学报(医学版), 2011, 43(2): 209-212. |
| [10] | 李玉慧, 姚海红, 张学武, 栗占国. 94例皮肌炎患者器官受累及免疫学特征分析[J]. 北京大学学报(医学版), 2010, 42(2): 140-142. |
| [11] | 沈光莉, 张巍, 李漪, 吕鹤, 袁云. 骨骼肌弥漫性钙化伴随皮下囊肿一例报告[J]. 北京大学学报(医学版), 2005, 37(6): 659-660. |
|
||