北京大学学报(医学版) ›› 2022, Vol. 54 ›› Issue (1): 40-47. doi: 10.19723/j.issn.1671-167X.2022.01.007
海马参与雌激素加重咬合干扰致去卵巢大鼠慢性咬肌痛敏
- 北京大学口腔医学院·口腔医院修复科,国家口腔医学中心,国家口腔疾病临床医学研究中心,口腔数字化医疗技术和材料国家工程实验室,口腔数字医学北京市重点实验室,国家卫生健康委员会口腔医学计算机应用工程技术研究中心,国家药品监督管理局口腔生物材料重点实验室,北京 100081
Hippocampus is involved in 17β-estradiol exacerbating experimental occlusal inter-ference-induced chronic masseter hyperalgesia in ovariectomized rats
FAN Ying-ying,LIU Yun,CAO Ye( ),XIE Qiu-fei(
),XIE Qiu-fei( )
)
- Department of Prosthodontics, Peking University School and Hospital of Stomatology & National Center of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology & NHC Research Center of Engineering and Technology for Computerized Dentistry & NMPA Key Laboratory for Dental Materials, Beijing 100081, China
摘要:
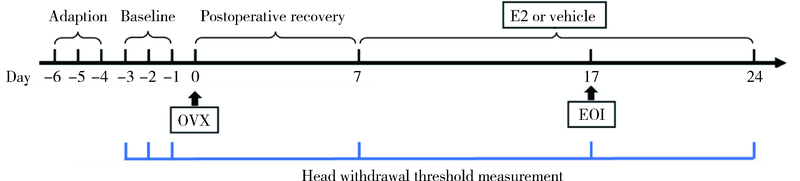
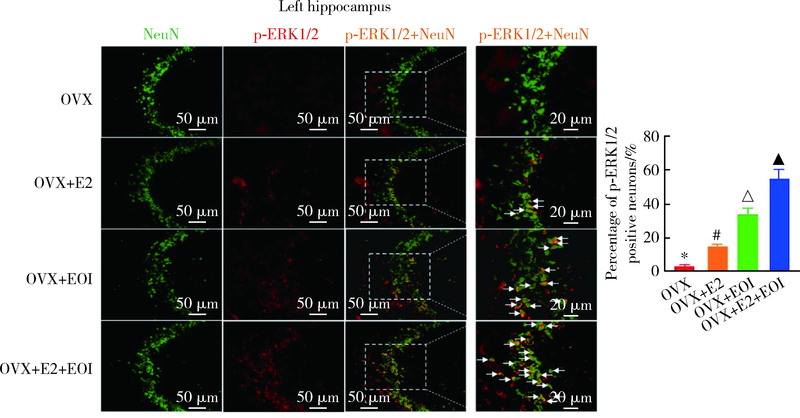
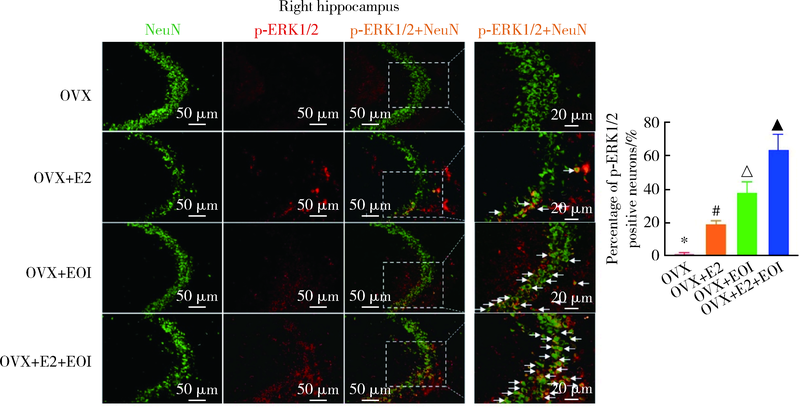
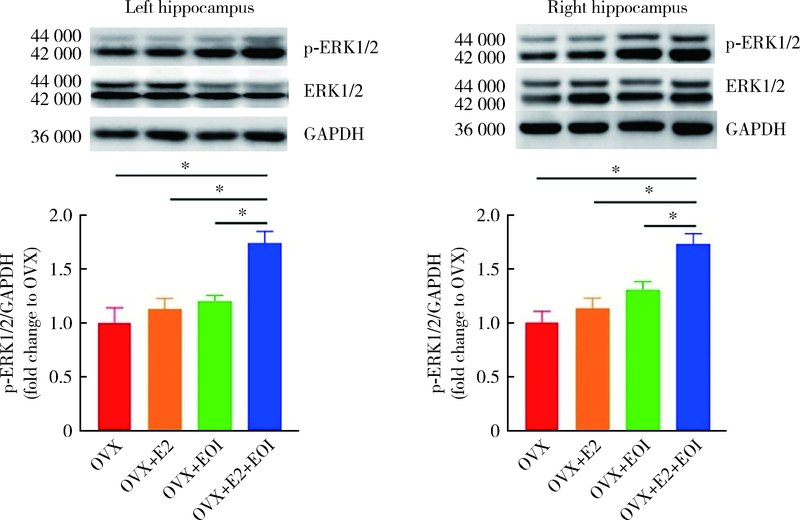
目的: 探究海马参与雌激素(主要为17β-雌二醇,17β-estradiol,E2)和实验性咬合干扰(experimental occlusal interference,EOI)对双侧卵巢摘除(ovariectomy,OVX)大鼠慢性咬肌痛敏影响的分子机制。方法: 将32只OVX大鼠随机分为4组(8只/组),对照组: OVX组,于OVX术后7 d开始0 μg/d E2(即用溶剂vehicle替代),不施加EOI;实验组:(1)OVX+E2组,于OVX术后7 d开始80 μg/d E2替代,不施加EOI;(2)OVX+EOI组,于OVX术后7 d开始0 μg/d E2替代,术后17 d施加EOI;(3)OVX+E2+EOI组,于OVX术后7 d开始80 μg/d E2替代,术后17 d施加EOI。于OVX术前、术后7 d(E2替代开始)、术后17 d(E2替代10 d,即EOI开始)、术后24 d(EOI 7 d)分别测试机械刺激反应阈值,建模完成后取大鼠双侧海马进行免疫荧光染色,观察海马CA3区神经元磷酸化细胞外信号调节激酶(phospho-extracellular signal regulated kinase 1/2,p-ERK1/2)表达情况,以及取大鼠双侧海马进行Western Blot定量检测p-ERK1/2表达量。结果: 与对照组[左侧:(135.3±8.5) g,右侧:(135.4±10.8) g]相比,OVX+E2组[左侧:(113.3±5.6) g,右侧:(112.5±5.6) g]和OVX+EOI组[左侧:(93.3±5.4) g,右侧:90.8±5.5) g]大鼠双侧咬肌机械刺激反应阈值显著降低(P<0.01);OVX+E2+EOI组[左侧:(81.2±6.2) g,右侧:79.8±7.7) g]阈值降低较对照组、OVX+E2组和OVX+EOI组显著(P<0.05);对照组、OVX+E2组、OVX+EOI组和OVX+E2+EOI组大鼠双侧海马CA3区p-ERK1/2阳性神经元比例依次增高,且组间差异有统计学意义(P<0.05);p-ERK1/2蛋白表达量在对照组、OVX+E2组、OVX+EOI组依次增高,组间差异无统计学意义(P > 0.05),OVX+E2+EOI组p-ERK1/2表达量显著高于其他3组(P<0.05)。结论: 高浓度E2可加重EOI导致的OVX大鼠慢性咬肌痛敏,其中枢机制可能与海马内ERK1/2信号通路有关。
中图分类号:
- R781.6
| [1] | Cao Y. Occlusal disharmony and chronic oro-facial pain: from clinical observation to animal study[J]. J Oral Rehabil, 2021, 7 (2021-07-17) [2021-09-19]. https://onlinelibrary.wiley.com/doi/epdf/10.1111/joor.13236 . |
| [2] | Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon[J]. Nat Rev Neuro-sci, 2012, 13(12):859-866. |
| [3] |
Cairns BE. The influence of gender and sex steroids on craniofacial nociception[J]. Headache, 2007, 47(2):319-324.
pmid: 17300382 |
| [4] |
Bueno CH, Pereira DD, Pattussi MP, et al. Gender differences in temporomandibular disorders in adult populational studies: a systematic review and meta-analysis[J]. J Oral Rehabil, 2018, 45(9):720-729.
doi: 10.1111/joor.12661 pmid: 29851110 |
| [5] | Slade GD, Bair E, By K, et al. Study methods, recruitment, sociodemographic findings, and demographic representativeness in the OPPERA study[J]. J Pain, 2011, 12(11):12-26. |
| [6] |
Castrillon EE, Cairns BE, Wang K, et al. Comparison of glutamate-evoked pain between the temporalis and masseter muscles in men and women[J]. Pain, 2012, 153(4):823-829.
doi: 10.1016/j.pain.2012.01.003 pmid: 22336721 |
| [7] |
Cao Y, Xie QF, Li K, et al. Experimental occlusal interference induces long-term masticatory muscle hyperalgesia in rats[J]. Pain, 2009, 144(3):287-293.
doi: 10.1016/j.pain.2009.04.029 pmid: 19473767 |
| [8] | Liu Y, Zhang XY, Fan YY, et al. Genistein reverses the effect of 17β-estradiol on exacerbating experimental occlusal interference-induced chronic masseter hyperalgesia in ovariectomised rats[J]. J Oral Rehabil, 2021, 2021, 6 (2021-06-02) [2021-09-19]. . |
| [9] | Niu K, Saloman JL, Zhang Y, et al. Sex differences in the contribution of ATP-sensitive K(+) channels in trigeminal ganglia under an acute muscle pain condition[J]. Neuroscience, 2011, 1809(4):344-352. |
| [10] |
Cairns BE, Hu JW, Arendt-Nielsen L, et al. Sex-related differences in human pain and rat afferent discharge evoked by injection of glutamate into the masseter muscle[J]. J Neurophysiol, 2001, 86(2):782-791.
pmid: 11495950 |
| [11] |
Wood PB, Ledbetter CR, Glabus MF, et al. Hippocampal meta-bolite abnormalities in fibromyalgia: correlation with clinical features[J]. J Pain, 2009, 10(1):47-52.
doi: 10.1016/j.jpain.2008.07.003 |
| [12] | Shimo K, Ueno T, Younger J, et al. Visualization of painful experiences believed to trigger the activation of affective and emotional brain regions in subjects with low back pain[J]. PLoS One, 2011, 6(11):1-6. |
| [13] |
Sakiyama Y, Sato A, Senda M, et al. Positron emission tomography reveals changes in global and regional cerebral blood flow during noxious stimulation of normal and inflamed elbow joints in anesthetized cats[J]. Exp Brain Res, 1998, 118(4):439-446.
doi: 10.1007/s002210050300 |
| [14] |
Derbyshire SWG, Jones AKP, Gyulai F, et al. Pain processing during three levels of noxious stimulation produces differential patterns of central activity[J]. Pain, 1997, 73(3):431-445.
pmid: 9469535 |
| [15] |
Aloisi AM, Zimmermann M, Herdegen T. Sex-dependent effects of formalin and restraint on c-Fos expression in the septum and hippocampus of the rat[J]. Neuroscience, 1997, 81(4):951-958.
pmid: 9330358 |
| [16] |
Jia M, Dahlman-Wright K, Gustafsson JÅ. Estrogen receptor alpha and beta in health and disease[J]. Best Pract Res Clin Endocrinol Metab, 2015, 29(4):557-568.
doi: 10.1016/j.beem.2015.04.008 |
| [17] |
Warfvinge K, Krause DN, Maddahi A, et al. Estrogen receptors α, β and GPER in the CNS and trigeminal system-molecular and functional aspects[J]. J Headache Pain, 2020, 21(1):131.
doi: 10.1186/s10194-020-01197-0 |
| [18] |
Prange-Kiel J, Rune GM. Direct and indirect effects of estrogen on rat hippocampus[J]. Neuroscience, 2006, 138(3):765-772.
pmid: 16324798 |
| [19] |
Henderson LA, Gandevia SC, Macefield VG. Gender differences in brain activity evoked by muscle and cutaneous pain: A retrospective study of single-trial fMRI data[J]. Neuroimage, 2008, 39(4):1867-1876.
pmid: 18069004 |
| [20] | Hubbard CS, Karpowicz JM, Furman AJ, et al. Estrogen-depen-dent visceral hypersensitivity following stress in rats: an fMRI study[J]. Mol Pain, 2016, 12:1-10. |
| [21] |
Jie HF, Yang GJ, Bi RY, et al. Genistein antagonizes 17β-estradiol effects on glutamate-evoked masseter muscle hypernociception in rats[J]. Front Neurol, 2018, 9:649.
doi: 10.3389/fneur.2018.00649 |
| [22] | Liverman CS, Brown JW, Sandhir R, et al. Oestrogen increases nociception through ERK activation in the trigeminal ganglion: evidence for a peripheral mechanism of allodynia[J]. Cephalal-gia, 2009, 29(5):520-531. |
| [23] |
Ji RR, Kohno T, Moore KA, et al. Central sensitization and LTP: do pain and memory share similar mechanisms?[J]. Trends Neurosci, 2003, 26(12):696-705.
doi: 10.1016/j.tins.2003.09.017 |
| [24] |
Martuscello RT, Spengler RN, Bonoiu AC, et al. Increasing TNF levels solely in the rat hippocampus produces persistent pain-like symptoms[J]. Pain, 2012, 153(9):1871-1882.
doi: 10.1016/j.pain.2012.05.028 pmid: 22770843 |
| [25] |
Ding TT, Xu XX, Cao Y, et al. Inflammatory pain memory facilitates occlusal interference-induced masticatory muscle hyperalgesia in rats[J]. Eur J Pain, 2016, 20(3):353-364.
doi: 10.1002/ejp.730 pmid: 26014463 |
| [26] |
Gurtskaia G, Tsiklauri N, Nozadze I, et al. Antinociceptive tolerance to NSAIDs microinjected into dorsal hippocampus[J]. BMC Pharmacol Toxicol, 2014, 15(1):10.
doi: 10.1186/2050-6511-15-10 |
| [27] |
Mckenna JE, Melzack R. Blocking NMDA receptors in the hippocampal dentate gyrus with AP5 produces analgesia in the formalin pain test[J]. Exp Neurol, 2001, 172(1):92-99.
doi: 10.1006/exnr.2001.7777 |
| [28] |
Gol A, Faibish GM. Effects of human hippocampal ablation[J]. J Neurosurg, 1967, 26(4):390.
pmid: 6021342 |
| [29] |
Liu Y, Xu XX, Cao Y, et al. 17β-Estradiol exacerbated experimental occlusal interference-induced chronic masseter hyperalgesia by increasing the neuronal excitability and TRPV1 function of trigeminal ganglion in ovariectomized rats[J]. Int J Mol Sci, 2021, 22(13):6945.
doi: 10.3390/ijms22136945 |
| [30] | Bi RY, Meng Z, Zhang P, et al. Estradiol upregulates voltage-gated sodium channel 1.7 in trigeminal ganglion contributing to hyperalgesia of inflamed TM[J]. PLoS One, 2017, 12(6):1-19. |
| [31] |
Payrits M, Sághy é, Cseko K, et al. Estradiol sensitizes the transient receptor potential vanilloid 1 receptor in pain responses[J]. Endocrinology, 2017, 158(10):3249-3258.
doi: 10.1210/en.2017-00101 pmid: 28977586 |
| [32] | 徐啸翔, 曹烨, 傅开元, 等. 咬合干扰致大鼠咬肌能量代谢产物含量变化[J]. 北京大学学报(医学版), 2017, 49(1):25-30. |
| [33] | 徐啸翔, 曹烨, 丁婷婷, 等. 三叉神经节嘌呤能P2X4受体参与牙合干扰致大鼠咬肌痛觉过敏的研究[J]. 中华口腔医学杂志, 2016, 51(3):176-181. |
| [34] |
Xu XX, Cao Y, Ding TT, et al. Role of TRPV1 and ASIC3 channels in experimental occlusal interference-induced hyperalgesia in rat masseter muscle[J]. Eur J Pain, 2016, 20(4):552-563.
doi: 10.1002/ejp.758 pmid: 26201614 |
| [35] |
Chen Y, Chen AQ, Luo XQ, et al. Hippocampal NR2B-containing NMDA receptors enhance long-term potentiation in rats with chronic visceral pain[J]. Brain Res, 2014, 1570:43-53.
doi: 10.1016/j.brainres.2014.05.001 pmid: 24824341 |
| [36] | Simonic-Kocijan S, Zhao X, Liu W, et al. TRPV1 channel-mediated bilateral allodynia induced by unilateral masseter muscle inflammation in rats[J]. Mol Pain, 2013, 9(1):68. |
| [37] |
Kim MT, Soussou W, Gholmieh G, et al. 17beta-Estradiol potentiates field excitatory postsynaptic potentials within each subfield of the hippocampus with greatest potentiation of the associational/commissural afferents of CA3[J]. Neuroscience, 2006, 141(1):391.
pmid: 16725270 |
| [38] | 贾静. 大鼠三叉神经脊束核和海马星形胶质细胞对咬合创伤的反应[D]. 北京, 中国人民解放军军医进修学院, 2005. |
| [39] |
Wu YW, Kou XX, Bi RY, et al. Hippocampal nerve growth factor potentiated by 17β-estradiol and involved in allodynia of inflamed TMJ in rat[J]. J Pain, 2012, 13(6):555-563.
doi: 10.1016/j.jpain.2012.03.005 |
| [40] |
Leresche L, Saunders K, von Korff MR, et al. Use of exogenous hormones and risk of temporomandibular disorder pain[J]. Pain, 1997, 69(1/2):153-160.
doi: 10.1016/S0304-3959(96)03230-7 |
| [1] | 闫树东,杨广聚,莫思怡,刘云,谢秋菲. 大鼠后肢长期抗阻训练对慢性咬肌机械痛觉敏感性的影响[J]. 北京大学学报(医学版), 2019, 51(1): 21-27. |
| [2] | 涂静宜, 朱莹, 尚淑玲, 张茜, 唐慧, 王瑞敏. Keap1-tat小肽降低缺血后大鼠海马CA1区神经元氧化应激损伤和空间学习记忆缺陷[J]. 北京大学学报(医学版), 2016, 48(1): 154-159. |
| [3] | 谭宏宇, , 叶铁虎, 马士平, 王晓良. 异丙酚对大鼠海马神经元低电压激活钙电流的抑制作用[J]. 北京大学学报(医学版), 2011, 43(2): 234-237. |
| [4] | 谭宏宇, , 孙丽娜, 王晓良, 叶铁虎 . 氯胺酮对大鼠海马神经元瞬间外向钾电流的抑制作用[J]. 北京大学学报(医学版), 2010, 42(2): 179-182. |
| [5] | 韩颖, 秦炯, 常杏芝, 杨志仙, 杜军保. 反复热性惊厥前后硫化氢/胱硫醚-β-合成酶体系表达的改变[J]. 北京大学学报(医学版), 2005, 37(6): 579-581. |
| [6] | 王丽珺, 胡白和, 何其华, 樊景禹. 乙酰胆碱对大鼠海马神经细胞内钙信号及细胞间通讯的影响[J]. 北京大学学报(医学版), 2004, 36(4): 357-360. |
| [7] | 单英, 秦炯, 常杏芝, 杨志仙. 纳洛酮对幼年大鼠反复热惊厥后远期惊厥易感性的影响[J]. 北京大学学报(医学版), 2004, 36(1): 57-60. |
| [8] | 韩颖, 秦炯, 常杏芝, 杨志仙. 发育期大鼠高热惊厥前后海马γ-氨基丁酸B受体亚基表达的变化[J]. 北京大学学报(医学版), 2003, 35(3): 288-291. |
| [9] | 姜玉武, 张国军, 吴希如. 发育期大鼠三氟乙醚致反复惊厥后海马NMDA受体亚基表达变化[J]. 北京大学学报(医学版), 2002, 34(3): 229-233. |
| [10] | 杨志仙, 秦炯, 周国平, 常杏芝. 发育期大鼠高热惊厥脑损伤模型的建立[J]. 北京大学学报(医学版), 2002, 34(3): 225-228. |
| [11] | 赵世刚, 罗强, 吴希如. 遗传性癫疒间易感大鼠P77PMC海马苔藓状纤维发芽的研究[J]. 北京大学学报(医学版), 2001, 33(2): 101-104. |
|
||