北京大学学报(医学版) ›› 2022, Vol. 54 ›› Issue (1): 31-39. doi: 10.19723/j.issn.1671-167X.2022.01.006
氧化锆多孔表面显微形貌对成骨细胞增殖及分化的影响
王铮1,丁茜1,2,△( ),高远1,马全诠1,张磊1,△(
),高远1,马全诠1,张磊1,△( ),葛兮源3,孙玉春1,4,谢秋菲1
),葛兮源3,孙玉春1,4,谢秋菲1
- 1.北京大学口腔医学院·口腔医院修复科,国家口腔医学中心,国家口腔疾病临床医学研究中心,口腔数字化医疗技术和材料国家工程实验室,口腔数字医学北京市重点实验室,国家卫生健康委员会口腔医学计算机应用工程技术研究中心,国家药品监督管理局口腔生物材料重点实验室,北京 100081
2.佛山(华南)新材料研究院,广东佛山 528000
3.北京大学口腔医学院·口腔医院中心实验室,北京 100081
4.北京大学口腔医学院·口腔医院口腔医学数字化研究中心,北京 100081
Effect of porous zirconia ceramics on proliferation and differentiation of osteoblasts
WANG Zheng1,DING Qian1,2,△( ),GAO Yuan1,MA Quan-quan1,ZHANG Lei1,△(
),GAO Yuan1,MA Quan-quan1,ZHANG Lei1,△( ),GE Xi-yuan3,SUN Yu-chun1,4,XIE Qiu-fei1
),GE Xi-yuan3,SUN Yu-chun1,4,XIE Qiu-fei1
- 1. Department of Prosthodontics, Peking University School and Hospital of Stomatology & National Center of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology & NHC Research Center of Engineering and Technology for Computerized Dentistry & NMPA Key Laboratory for Dental Materials, Beijing 100081, China
2. Foshan (Southern China) Institute for New Materials, Foshan 528000, Guangdong, China
3. Central Laboratory, Peking University School and Hospital of Stomatology, Beijing 100081, China
4. Center for Digital Dentistry, Peking University School and Hospital of Stomatology, Beijing 100081, China
摘要:
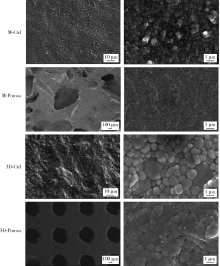
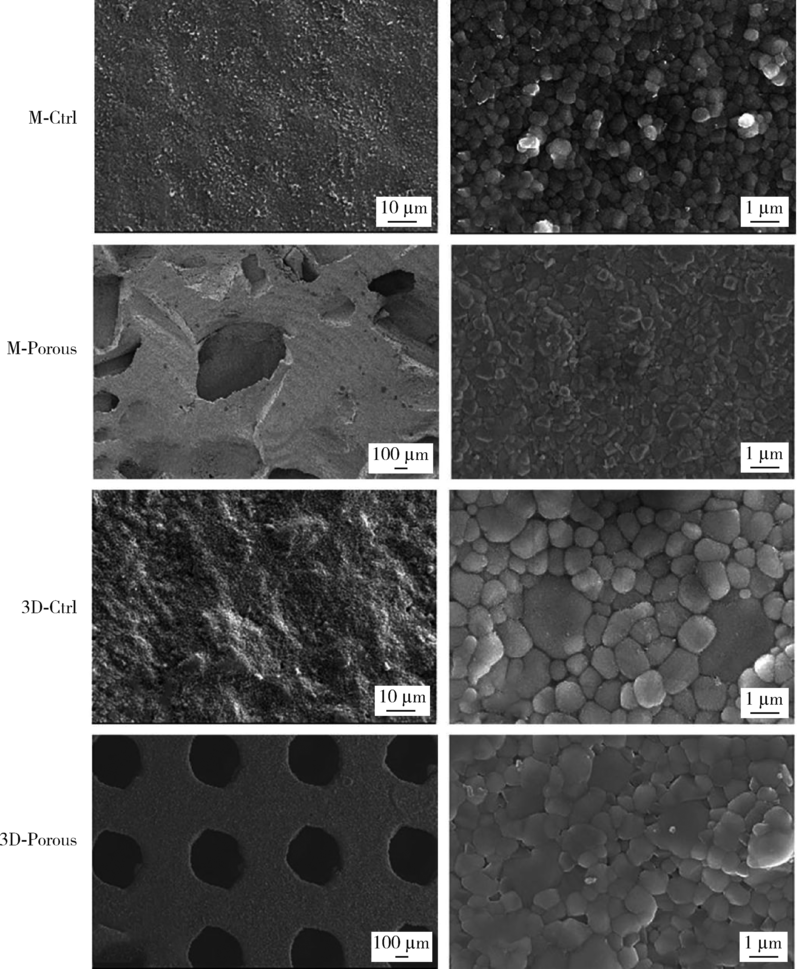
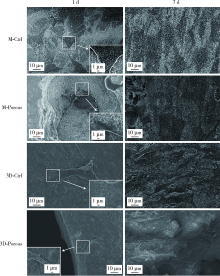
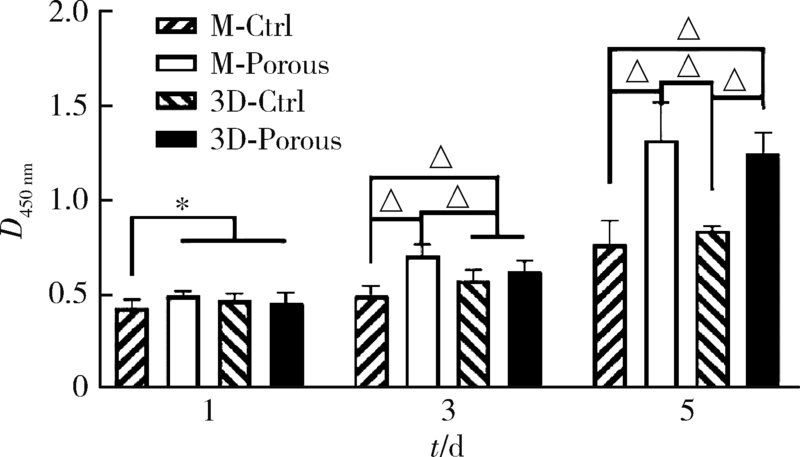
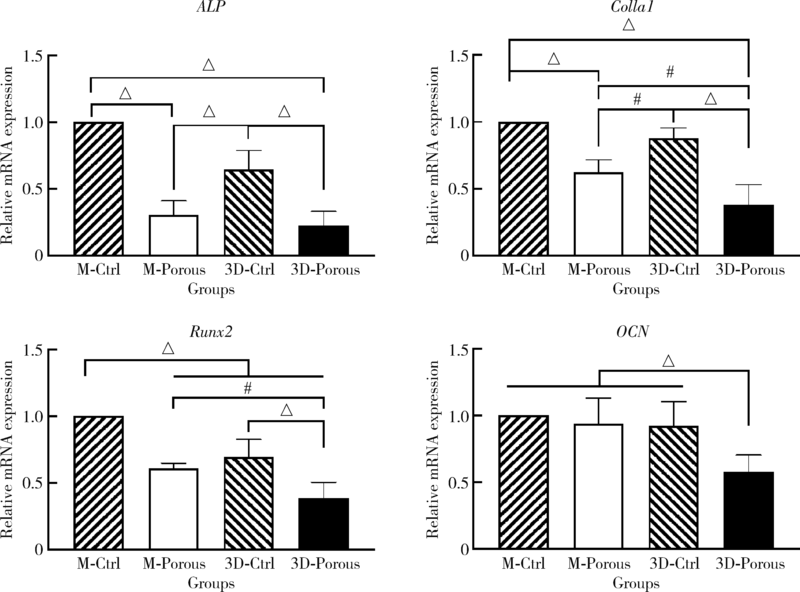
目的: 探索两种氧化锆多孔表面显微形貌对小鼠前成骨细胞增殖、分化的影响。方法: 根据加工及造孔方式不同,将氧化锆试件分为4组,分别为切削终烧结组(milled sintering group, M-Ctrl)、切削多孔组(milled porous group, M-Porous)、3D打印终烧结组(3D printed sintering group, 3D-Ctrl)和3D打印多孔组(3D printed porous group, 3D-Porous)。通过扫描电镜(scanning electron microscope, SEM)、激光显微形貌测量显微镜进行表面显微形貌分析,用接触角测量仪测量静态接触角,通过能量色散X射线仪进行表面元素分析。将小鼠胚胎成骨细胞前体细胞MC3T3-E1接种于试件表面,SEM观察细胞培养第1、7天的黏附形态;使用细胞增殖-毒性检测试剂盒(cell counting kit-8, CCK-8)测量细胞培养第1、3、5天的增殖情况;采用实时荧光定量聚合酶链式反应检测分化诱导第14天时碱性磷酸酶(alkaline phosphatase, ALP)、Ⅰ型胶原(type Ⅰ collagen, Colla1)、Runt相关转录因子2(Runt-related transcription factor-2, Runx2)及骨钙素(osteocalcin, OCN ) mRNA的相对表达量。结果: 3D-Porous组孔径[(419.72±6.99) μm]及孔深度[(560.38±8.55) μm]均显著大于M-Porous组的孔径[(300.55±155.65) μm]及孔深度[(69.97±31.38) μm,(P<0.05)], 且3D-Porous组圆孔形状更规则,分布更均匀。各组静态接触角均小于90°,其中3D-Ctrl组的静态接触角(73.83°±5.34°)与M-Porous组(72.7°±2.72°)最大,两组间差异无统计学意义(P>0.05)。M-Porous及3D-Porous组表面均可见细胞在孔内黏附,且培养第3、5天时两组试件表面细胞增殖活性均显著高于M-Ctrl组及3D-Ctrl组(P<0.05)。细胞分化诱导第14天时3D-Porous组ALP、Colla1、Runx2、OCN mRNA的相对表达量均显著低于M-Ctrl组及3D-Ctrl组(P < 0.05)。M-Porous组Colla1、Runx2、OCN mRNA的相对表达量均显著高于3D-Porous组(P<0.05)。结论: 氧化锆表面多孔形貌能促进小鼠前成骨细胞MC3T3-E1的增殖及黏附,但对其成骨向分化有一定抑制作用。
中图分类号:
- R781.3
| [1] |
Hanawa T. Zirconia versus titanium in dentistry: a review[J]. Dent Mater J, 2020, 39(1):24-36.
doi: 10.4012/dmj.2019-172 pmid: 31666488 |
| [2] |
Yoshinari M. Future prospects of zirconia for oral implants: a review[J]. Dent Mater J, 2020, 39(1):37-45.
doi: 10.4012/dmj.2019-151 pmid: 31666487 |
| [3] |
Roehling S, Astasov-Frauenhoffer M, Hauser-Gerspach I, et al. In vitro biofilm formation on titanium and zirconia implant surfaces[J]. J Periodontol, 2017, 88(3):298-307.
doi: 10.1902/jop.2016.160245 pmid: 27712464 |
| [4] |
Hafezeqoran A, Koodaryan R. Effect of zirconia dental implant surfaces on bone integration: a systematic review and meta analysis[J]. Biomed Res Int, 2017, 2017:9246721.
doi: 10.1155/2017/9246721 pmid: 28299337 |
| [5] |
Cionca N, Hashim D, Mombelli A. Zirconia dental implants: where are we now, and where are we heading?[J]. Periodontol 2000, 2017, 73(1):241-258.
doi: 10.1111/prd.12180 |
| [6] |
Adanez MH, Nishihara H, Att W. A systematic review and meta analysis on the clinical outcome of zirconia implant restoration complex[J]. J Prosthodont Res, 2018, 62(4):397-406.
doi: 10.1016/j.jpor.2018.04.007 |
| [7] | Roehling S, Schlegel KA, Woelfler H, et al. Zirconia compared to titanium dental implants in preclinical studies: a systematic review and meta analysis[J]. Clin Oral Implants Res, 2019, 30(5):365-395. |
| [8] |
Kohal RJ, Bachle M, Att W, et al. Osteoblast and bone tissue response to surface modified zirconia and titanium implant materials[J]. Dent Mater, 2013, 29(7):763-776.
doi: 10.1016/j.dental.2013.04.003 pmid: 23669198 |
| [9] |
El-Hadad S, Safwat EM, Sharaf NF. In vitro and in vivo, cytoto-xicity evaluation of cast functionally graded biomaterials for dental implantology[J]. Mater Sci Eng C Mater Biol Appl, 2018, 93:987-995.
doi: 10.1016/j.msec.2018.09.003 |
| [10] |
Shirazi HA, Ayatollahi MR, Asnafi A. To reduce the maximum stress and the stress shielding effect around a dental implant bone interface using radial functionally graded biomaterials[J]. Comput Methods Biomech Biomed Engin, 2017, 20(7):750-759.
doi: 10.1080/10255842.2017.1299142 |
| [11] |
Dele-Afolabi TT, Hanim MAA, Norkhairunnisa M, et al. Research trend in the development of macroporous ceramic components by pore forming additives from natural organic matters: a short review[J]. Ceram Int, 2017, 43(2):1633-1649.
doi: 10.1016/j.ceramint.2016.10.177 |
| [12] |
Hadjicharalambous C, Mygdali E, Prymak O, et al. Proliferation and osteogenic response of MC3T3-E1 preosteoblastic cells on porous zirconia ceramics stabilized with magnesia or yttria[J]. J Biomed Mater Res A, 2015, 103(11):3612-3624.
doi: 10.1002/jbm.a.35475 pmid: 25847599 |
| [13] |
Prymak O, Vagiaki LE, Buyakov A, et al. Porous zirconia/magnesia ceramics support osteogenic potential in vitro[J]. Materials (Basel), 2021, 14(4):1049.
doi: 10.3390/ma14041049 |
| [14] | 王艳芬, 牛光良, 韩建民. 氧化锆微米涂层对成骨细胞增殖和分化的影响[J]. 中华口腔医学杂志, 2018, 53(5):339-343. |
| [15] | Ali N, Safwat A, Aboushelib M. The effect of fusion sputtering surface treatment on microshear bond strength of zirconia and MDP containing resin cement[J]. Dent Mater, 2019, 35(6):107-112. |
| [16] |
Liu Y, Rath B, Tingart M, et al. Role of implants surface modification in osseointegration: a systematic review[J]. J Biomed Mater Res A, 2020, 108(3):470-484.
doi: 10.1002/jbm.a.36829 pmid: 31664764 |
| [17] |
Sakthiabirami K, Kang JH, Jang JG, et al. Hybrid porous zirconia scaffolds fabricated using additive manufacturing for bone tissue engineering applications[J]. Mater Sci Eng C Mater Biol Appl, 2021, 123:111950.
doi: 10.1016/j.msec.2021.111950 |
| [18] |
Hwa LC, Rajoo S, Noor AM, et al. Recent advances in 3D prin-ting of porous ceramics: a review[J]. Curr Opin Solid State Mater Sci, 2017, 21(6):323-347.
doi: 10.1016/j.cossms.2017.08.002 |
| [19] |
Li R, Chen H, Wang Y, et al. Performance of stereolithography and milling in fabricating monolithic zirconia crowns with different finish line designs[J]. J Mech Behav Biomed Mater, 2021, 115:104255.
doi: 10.1016/j.jmbbm.2020.104255 |
| [20] |
Chen ZW, Li ZY, Li JJ, et al. 3D printing of ceramics: a review[J]. J Eur Ceram Soc, 2019, 39(4):661-687.
doi: 10.1016/j.jeurceramsoc.2018.11.013 |
| [21] |
Zaharin HA, Rani AMA, Azam FI, et al. Effect of unit cell type and pore size on porosity and mechanical behavior of additively manufactured Ti6Al4V scaffolds[J]. Materials (Basel), 2018, 11(12):2402.
doi: 10.3390/ma11122402 |
| [22] |
Arabnejad S, Johnston RB, Pura JA, et al. High-strength porous biomaterials for bone replacement: a strategy to assess the interplay between cell morphology, mechanical properties, bone ingrowth and manufacturing constraints[J]. Acta Biomater, 2016, 30:345-356.
doi: S1742-7061(15)30177-X pmid: 26523335 |
| [23] |
Bael SV, Chai YC, Truscello S, et al. The effect of pore geometry on the in vitro biological behavior of human periosteum derived cells seeded on selective laser melted Ti6Al4V bone scaffolds[J]. Acta Biomater, 2012, 8(7):2824-2834.
doi: 10.1016/j.actbio.2012.04.001 pmid: 22487930 |
| [24] |
Wang XJ, Xu SQ, Zhou SW, et al. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: a review[J]. Biomaterials, 2016, 83:127-141.
doi: 10.1016/j.biomaterials.2016.01.012 |
| [25] |
Hadjicharalambous C, Buyakov A, Buyakova S, et al. Porous alumina, zirconia and alumina/zirconia for bone repair: fabrication, mechanical and in vitro biological response[J]. Biomed Mater, 2015, 10(2):025012.
doi: 10.1088/1748-6041/10/2/025012 |
| [26] |
Wang H, Su KX, Su LZ, et al. The effect of 3D-printed Ti6Al4V scaffolds with various macropore structures on osteointegration and osteogenesis: a biomechanical evaluation[J]. J Mech Behav Biomed Mater, 2018, 88:488-496.
doi: S1751-6161(18)30687-8 pmid: 30223212 |
| [27] | 赖颖真, 卢薛冠, 蔡艺煌. 钛及氧化锆表面微沟槽结构对人牙龈成纤维细胞生物学行为的影响[J]. 中华口腔医学杂志, 2019, 54(10):676-682. |
| [28] |
Wang C, Xu DL, Li SJ, et al. Effect of pore size on the physicochemical properties and osteogenesis of Ti6Al4V porous scaffolds with bionic structure[J]. ACS Omega, 2020, 5(44):28684-28692.
doi: 10.1021/acsomega.0c03824 |
| [29] |
Kapat K, Srivas PK, Rameshbabu AP, et al. Influence of porosity and pore size distribution in Ti6Al4V foam on physicomechanical properties, osteogenesis, and quantitative validation of bone ingrowth by micro computed tomography[J]. ACS Appl Mater Interfaces, 2017, 9(45):39235-39248.
doi: 10.1021/acsami.7b13960 |
| [30] |
Gitten RA, McLachlan T, Olivares-Navarrete R, et al. The effects of combined micron/submicron scale surface roughness and nanoscale features on cell proliferation and differentiation[J]. Biomaterials, 2011, 32(13):3395-3403.
doi: 10.1016/j.biomaterials.2011.01.029 |
| [31] |
Wang XK, Schwartz Z, Gittens RA, et al. Role of integrin alpha2 beta1 in mediating osteoblastic differentiation on three dimensional titanium scaffolds with submicron scale texture[J]. J Biomed Mater Res A, 2015, 103(6):1907-1918.
doi: 10.1002/jbm.a.v103.6 |
| [32] |
Lv J, Jia ZJ, Li J, et al. Electron beam melting fabrication of porous Ti6Al4V scaffolds: cytocompatibility and osteogenesis[J]. Adv Eng Mater, 2015, 17(9):1391-1398.
doi: 10.1002/adem.v17.9 |
| [33] |
Rezaei NM, Hasegawa M, Ishijima M, et al. Biological and osseointegration capabilities of hierarchically (meso/micro/nano scale) roughened zirconia[J]. Int J Nanomedicine, 2018, 13:3381-3395.
doi: 10.2147/IJN |
| [34] |
Mostafa D, Aboushelib M. Bioactive-hybrid-zirconia implant surface for enhancing osseointegration: an in vivo study[J]. Int J Implant Dent, 2018, 4(1):20.
doi: 10.1186/s40729-018-0129-3 pmid: 29900480 |
| [35] |
Xing HY, Zou B, Li SS, et al. Study on surface quality, precision and mechanical properties of 3D printed ZrO2 ceramic components by laser scanning stereolithography[J]. Ceram Int, 2017, 43(18):16340-16347.
doi: 10.1016/j.ceramint.2017.09.007 |
| [36] |
Zhu YL, Zhu RQ, Ma J, et al. In vitro cell proliferation evaluation of porous nano zirconia scaffolds with different porosity for bone tissue engineering[J]. Biomed Mater, 2015, 10(5):055009.
doi: 10.1088/1748-6041/10/5/055009 |
| [37] |
Seuba J, Deville S, Guizard C, et al. The effect of wall thickness distribution on mechanical reliability and strength in unidirectional porous ceramics[J]. Sci Technol Adv Mater, 2016, 17(1):128-135.
doi: 10.1080/14686996.2016.1140309 |
| [1] | 丁茜,李文锦,孙丰博,谷景华,林元华,张磊. 表面处理对氧化钇和氧化镁稳定的氧化锆种植体晶相及断裂强度的影响[J]. 北京大学学报(医学版), 2023, 55(4): 721-728. |
| [2] | 李伟伟,陈虎,王勇,孙玉春. 氧化锆陶瓷表面硅锂喷涂层的摩擦磨损性能[J]. 北京大学学报(医学版), 2023, 55(1): 94-100. |
| [3] | 李文锦,丁茜,原福松,孙丰博,郑剑桥,鲍蕊,张磊. 飞秒激光表面处理对氧化锆表面特征及弯曲强度的影响[J]. 北京大学学报(医学版), 2021, 53(4): 770-775. |
| [4] | 李峥,王霄,洪天配,王浩杰,高展翼,万蒙. 晚期糖基化终末产物抑制大鼠外周血单个核细胞及成骨细胞增殖的作用机制[J]. 北京大学学报(医学版), 2021, 53(2): 355-363. |
| [5] | 杨欣,李榕,叶红强,陈虎,王勇,周永胜,孙玉春. 不同刃状边缘补偿角度的两种氧化锆全瓷冠断裂强度的评价[J]. 北京大学学报(医学版), 2021, 53(2): 402-405. |
| [6] | 任茜,周建,王鸣刚,陈克明. 脉冲电磁场促进成骨细胞成熟分化依赖于初级纤毛-PI3K/AKT途径[J]. 北京大学学报(医学版), 2019, 51(2): 245-251. |
| [7] | 郑苗,詹凌璐,刘志强,李和平,谭建国. 不同等离子体处理氧化锆对人牙龈成纤维细胞黏附能力的影响[J]. 北京大学学报(医学版), 2019, 51(2): 315-320. |
| [8] | 张智勇,孟甜,陈全,刘文曙,陈宇寰. 种植体早期失败病例回顾性分析[J]. 北京大学学报(医学版), 2018, 50(6): 1088-1091. |
| [9] | 周团锋,王新知. 计算机辅助设计与制作的一体化氧化锆全瓷桩核5年临床观察[J]. 北京大学学报(医学版), 2018, 50(4): 680-684. |
| [10] | 焦洋, 王继德, 邓久鹏. 不同表面处理对氧化锆晶相结构及性能的影响[J]. 北京大学学报(医学版), 2018, 50(1): 49-52. |
| [11] | 廖宇,刘晓强,陈立,周建锋,谭建国. 不同表面处理方法对氧化锆与树脂水门汀粘接强度的影响[J]. 北京大学学报(医学版), 2018, 50(1): 53-57. |
| [12] | 崔新悦,佟岱,王新知,沈志坚. 对比切削与胶态成型工艺制作的氧化锆陶瓷的透光性与遮色效果[J]. 北京大学学报(医学版), 2018, 50(1): 85-90. |
| [13] | 甘洪全,王茜,张辉,刘鑫,邓华民,宋会平,王志强,李琪佳. RGD多肽修饰多孔钽对MG63成骨样细胞-钽界面形态及成骨因子表达的影响[J]. 北京大学学报(医学版), 2018, 50(1): 176-182. |
| [14] | 凌龙,赵玉鸣,葛立宏. 不同炎症状态下犬年轻恒牙牙髓干细胞增殖及成骨分化能力的改变[J]. 北京大学学报(医学版), 2016, 48(5): 878-883. |
| [15] | 周团锋, 张相皞, 王新知. 一体化计算机辅助设计与制作氧化锆桩核的三维有限元分析[J]. 北京大学学报(医学版), 2015, 47(1): 78-84. |
|
||