北京大学学报(医学版) ›› 2022, Vol. 54 ›› Issue (4): 599-604. doi: 10.19723/j.issn.1671-167X.2022.04.004
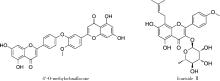
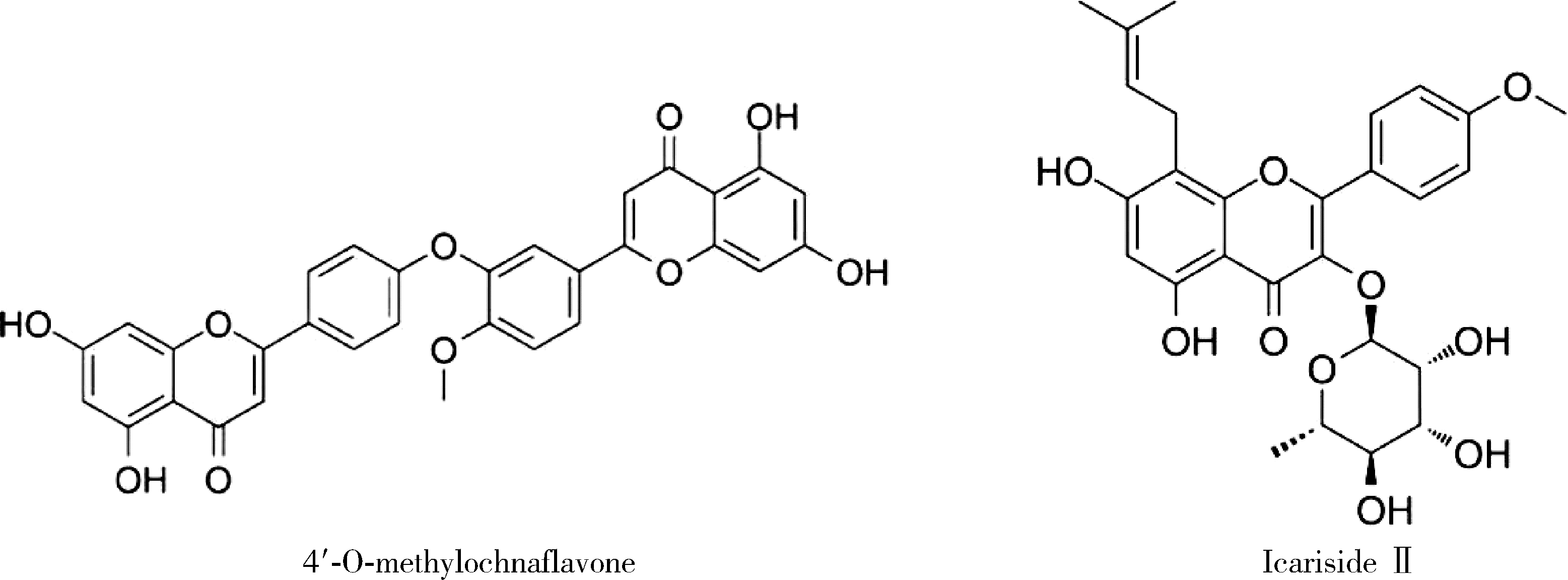
4′-甲基醚金连木黄酮对棕榈酸诱导的大鼠阴茎海绵体内皮细胞功能障碍的影响
顾阳阳1,谭晓辉1,宋文鹏1,方冬1,宋卫东1,袁亦铭1,冯宁翰2,*( ),关瑞礼1,*(
),关瑞礼1,*( )
)
- 1. 北京大学第一医院泌尿外科,北京大学泌尿外科研究所,北京 100034
2. 南京医科大学附属无锡第二医院泌尿外科,江苏无锡 214002
Effects of 4′-O-methylochnaflavone on endothelial dysfunction induced by palmitic acid in rat cavernous endothelial cells
Yang-yang GU1,Xiao-hui TAN1,Wen-peng SONG1,Dong FANG1,Wei-dong SONG1,Yi-ming YUAN1,Ning-han FENG2,*( ),Rui-li GUAN1,*(
),Rui-li GUAN1,*( )
)
- 1. Department of Urology, Peking University First Hospital; Institute of Urology, Peking University; Beijing 100034, China
2. Department of Urology, Wuxi Second Hospital, Nanjing Medical University, Wuxi 214002, Jiangsu, China
摘要:
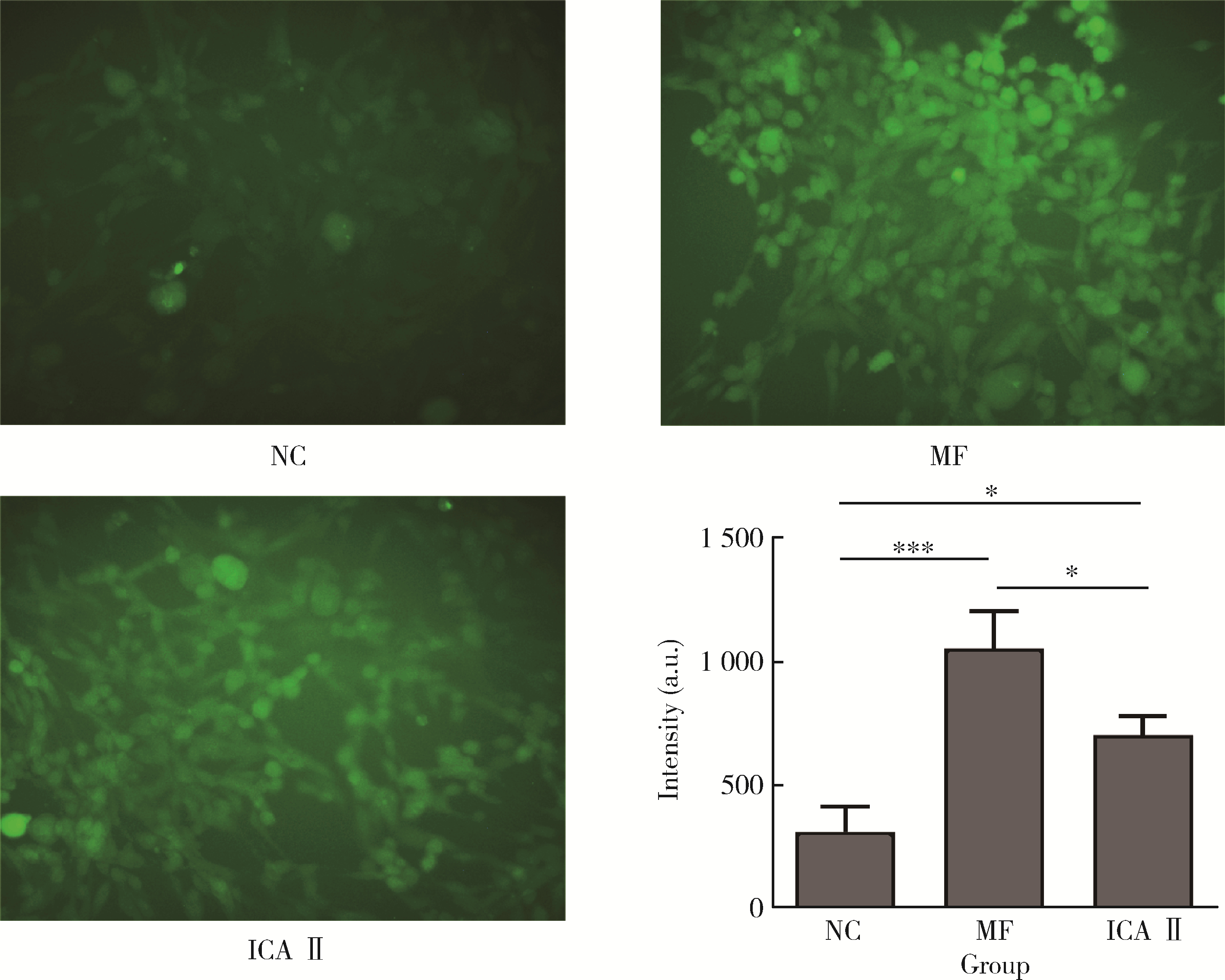
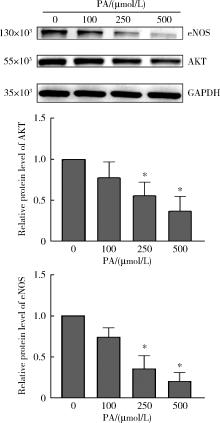
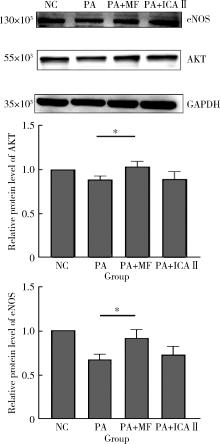
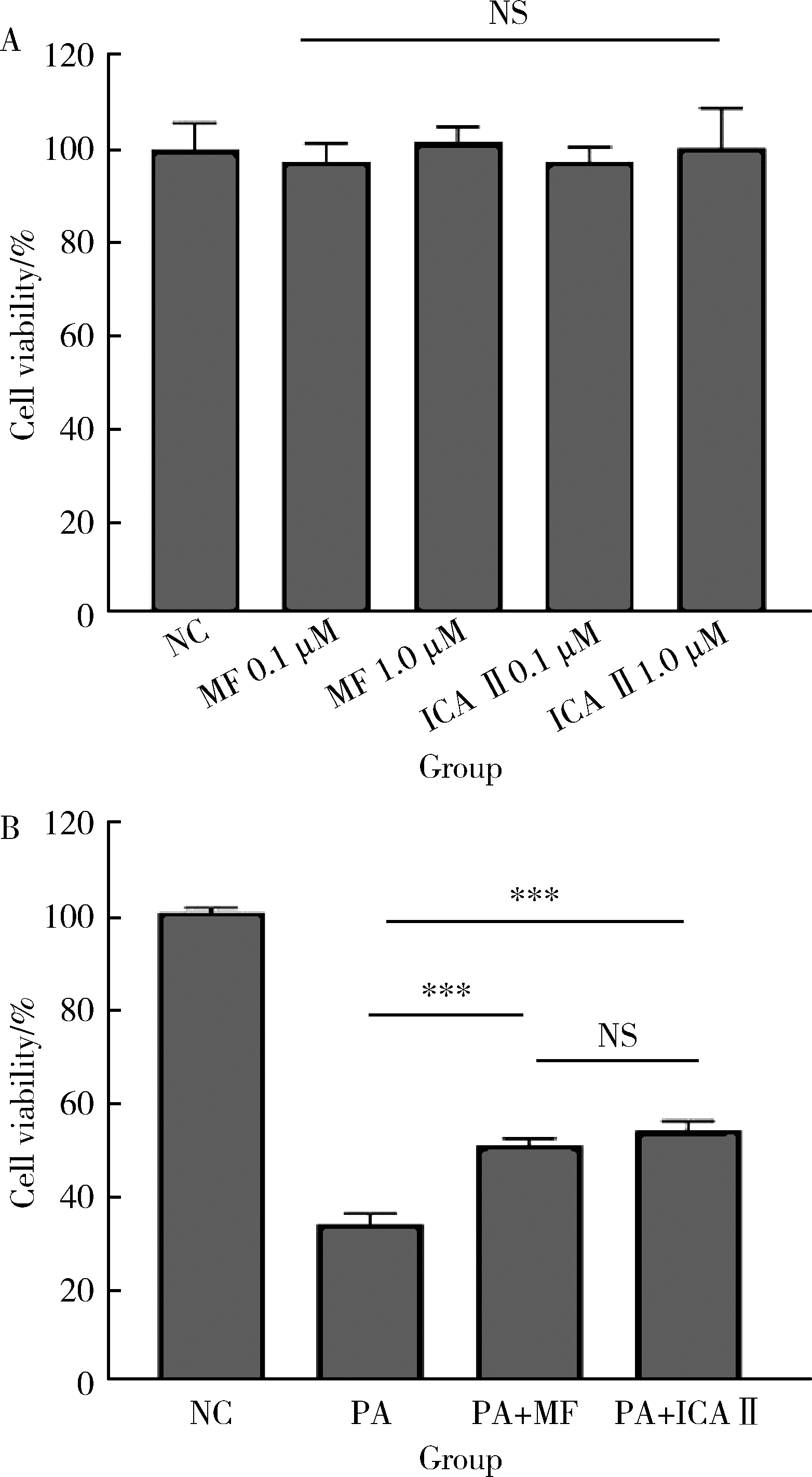
目的: 探讨双黄酮类药物4′-甲基醚金连木黄酮(4′-O-methylochnaflavone, MF)对棕榈酸诱导的大鼠阴茎海绵体内皮细胞(rat cavernous endothelial cells, RCECs)功能障碍的影响。方法: 将RCECs随机分为4组,分别为正常+牛血清白蛋白组(NC组)、棕榈酸(palmitic acid,PA)组、MF治疗组、淫羊藿次苷Ⅱ(icasiside Ⅱ,ICA Ⅱ)治疗组。采用蛋白印迹实验检测各组细胞的蛋白激酶B(protein kinase B, PKB/AKT)和内皮型一氧化氮合酶(endothelial nitric oxide synthase,eNOS)蛋白表达水平,使用一氧化氮荧光探针检测MF以及ICA Ⅱ对RCECs内一氧化氮含量的影响,使用CCK-8试剂盒检测MF、ICA Ⅱ对PA诱导后的RCECs增殖能力的影响。结果: 与NC组相比,MF组、ICA Ⅱ组处理后细胞内一氧化氮含量显著增加(P < 0.05),MF组的效果优于ICA Ⅱ组(P < 0.05)。与NC组相比,PA组的eNOS和AKT蛋白表达水平明显降低,提示成功构建了用于模拟高脂环境的体外RCECs内皮功能障碍模型(P < 0.05)。MF组干预后能够有效提高eNOS、AKT的表达水平,表明MF可促进恢复游离脂肪酸造成的内皮细胞损伤(P < 0.05)。CCK-8增殖实验显示PA显著降低RCECs的增殖数量(P < 0.05),而给予MF、ICA Ⅱ治疗后细胞增殖能力得到显著恢复(P < 0.05)。结论: 在RCECs中,MF与ICA Ⅱ能够有效增加细胞内一氧化氮含量,PA处理后AKT/eNOS通路的下调揭示其参与内皮细胞损伤的发生、发展,而MF的干预能够有效逆转上述变化,此外,PA诱导下RCECs的细胞增殖能力明显下降,而MF与ICA Ⅱ干预能够恢复上述变化。双黄酮类药物MF对PA诱导的RCECs细胞功能障碍有一定程度的修复作用。
中图分类号:
- R698.1
| 1 |
Bakr AM , El-Sakka AI . Erectile dysfunction among patients and health care providers during COVID-19 pandemic: A systematic review[J]. Int J Impot Res, 2022, 34 (2): 145- 151.
doi: 10.1038/s41443-021-00504-w |
| 2 |
Yafi FA , Jenkins L , Albersen M , et al. Erectile dysfunction[J]. Nat Rev Dis Primers, 2016, 2, 16003.
doi: 10.1038/nrdp.2016.3 |
| 3 |
Ghosh A , Gao L , Thakur A , et al. Role of free fatty acids in endothelial dysfunction[J]. J Biomed Sci, 2017, 24 (1): 50.
doi: 10.1186/s12929-017-0357-5 |
| 4 |
Zhao Y , Vanhoutte PM , Leung SWS . Vascular nitric oxide: Beyond eNOS[J]. J Pharmacol Sci, 2015, 129 (2): 83- 94.
doi: 10.1016/j.jphs.2015.09.002 |
| 5 |
Boden G . Obesity and free fatty acids[J]. Endocrin Metab Clin, 2008, 37 (3): 635- 646.
doi: 10.1016/j.ecl.2008.06.007 |
| 6 |
Egan BM , Greene EL , Goodfriend TL . Nonesterified fatty acids in blood pressure control and cardiovascular complications[J]. Curr Hypertens Rep, 2001, 3 (2): 107- 116.
doi: 10.1007/s11906-001-0021-y |
| 7 |
Haus JM , Solomon TPJ , Marchetti CM , et al. Free fatty acid-induced hepatic insulin resistance is attenuated following lifestyle intervention in obese individuals with impaired glucose tolerance[J]. J Clin Endocr Metab, 2010, 95 (1): 323- 327.
doi: 10.1210/jc.2009-1101 |
| 8 |
Durrant JR , Seals DR , Connell ML , et al. Voluntary wheel running restores endothelial function in conduit arteries of old mice: Direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase[J]. J Physiol, 2009, 587 (13): 3271- 3285.
doi: 10.1113/jphysiol.2009.169771 |
| 9 |
Khan MJ , Rizwan Alam M , Waldeck-Weiermair M , et al. Inhibition of autophagy rescues palmitic acid-induced necroptosis of endothelial cells[J]. J Biol Chem, 2012, 287 (25): 21110- 21120.
doi: 10.1074/jbc.M111.319129 |
| 10 |
Lee C , Lee S , Ou H , et al. Eicosapentaenoic acid protects against palmitic acid-induced endothelial dysfunction via activation of the AMPK/eNOS pathway[J]. Int J Mol Sci, 2014, 15 (6): 10334- 10349.
doi: 10.3390/ijms150610334 |
| 11 |
Wang L , Xu Y , Li H , et al. Antioxidant icariside Ⅱ combined with insulin restores erectile function in streptozotocin-induced type 1 diabetic rats[J]. J Cell Mol Med, 2015, 19 (5): 960- 969.
doi: 10.1111/jcmm.12480 |
| 12 |
Gu S J , Li M , Yuan YM , et al. A novel flavonoid derivative of icariside Ⅱ improves erectile dysfunction in a rat model of caver-nous nerve injury[J]. Andrology, 2021, 9 (6): 1893- 1901.
doi: 10.1111/andr.13065 |
| 13 | Godo S , Shimokawa H . Endothelial functions[J]. Arterioscler Thromb Vasc Biol, 2017, 37 (9): e108- e114. |
| 14 |
Chlopicki S . Perspectives in pharmacology of endothelium: From bench to bedside[J]. Pharmacol Rep, 2015, 67 (4): vi- ix.
doi: 10.1016/j.pharep.2015.08.005 |
| 15 |
Everaert BR , van Craenenbroeck EM , Hoymans VY , et al. Current perspective of pathophysiological and interventional effects on endothelial progenitor cell biology: Focus on Pi3K/AKT/eNOS pathway[J]. Int J Cardiol, 2010, 144 (3): 350- 366.
doi: 10.1016/j.ijcard.2010.04.018 |
| 16 |
Morris G , Puri BK , Olive L , et al. Endothelial dysfunction in neuroprogressive disorders-causes and suggested treatments[J]. BMC Med, 2020, 18 (1): 305.
doi: 10.1186/s12916-020-01749-w |
| 17 |
Mu H , Liu H , Zhang J , et al. Ursolic acid prevents doxorubicin-induced cardiac toxicity in mice through eNOS activation and inhibition of eNOS uncoupling[J]. J Cell Mol Med, 2019, 23 (3): 2174- 2183.
doi: 10.1111/jcmm.14130 |
| 18 | Schmitt CA , Dirsch VM . Modulation of endothelial nitric oxide by plant-derived products[J]. Nitric Oxide, 2009, 21 (2): 77- 91. |
| 19 |
García-Prieto CF , Hernández-Nuño F , Rio DD , et al. High-fat diet induces endothelial dysfunction through a down-regulation of the endothelial AMPK-PI3K-Akt-eNOS pathway[J]. Mol Nutr Food Res, 2015, 59 (3): 520- 532.
doi: 10.1002/mnfr.201400539 |
| 20 | Vadivel V , Kunyanga CN , Biesalski HK . Health benefits of nut consumption with special reference to body weight control[J]. Nutrition, 2012, 28 (11/12): 1089- 1097. |
| 21 |
Brookheart RT , Michel CI , Schaffer JE . As a matter of fat[J]. Cell Metab, 2009, 10 (1): 9- 12.
doi: 10.1016/j.cmet.2009.03.011 |
| 22 | Wang M , Gao H , Li W , et al. Icariin and its metabolites regulate lipid metabolism: From effects to molecular mechanisms[J]. Biomed Pharmacother, 2020, 131, 110675. |
| 23 | Li H , Xu Y , Guan R , et al. Icariside Ⅱ prevents high-glucose-induced injury on human cavernous endothelial cells through Akt-eNOS signaling pathway[J]. Andrology, 2015, 3 (2): 408- 416. |
| 24 | Lee S J , Choi JH , Son KH , et al. Suppression of mouse lymphocyte proliferation in vitro by naturally-occurring biflavonoids[J]. Life Sci, 1995, 57 (6): 551- 558. |
| 25 | Li L , Ma L , Hu Y , et al. Natural biflavones are potent inhibitors against SARS-CoV-2 papain-like protease[J]. Phytochemistry, 2022, 193, 112984. |
| [1] | 杨菁,杜娟,王玉湘,刘从容. JAK/STAT信号通路在卵巢高级别浆液性癌中的激活及预后意义[J]. 北京大学学报(医学版), 2023, 55(2): 270-275. |
| [2] | 董尔丹. 心血管受体的信号转导与疾病[J]. 北京大学学报(医学版), 2022, 54(5): 796-802. |
| [3] | 肖洋,杜瑶瑶,高成,孔炜. microRNA在高磷诱导血管平滑肌细胞钙化早期的动态变化[J]. 北京大学学报(医学版), 2016, 48(5): 756-765. |
| [4] | 刘涛, 覃新程, 李维仁, 周峰, 李广永, 辛华, 巩艳青, 辛钟成. 淫羊藿苷和淫羊藿次苷Ⅱ对内皮细胞eNOS表达和NOS活性的影响[J]. 北京大学学报(医学版), 2011, 43(4): 500-504. |
| [5] | 廖艳, 黎海芪, 邓兵, 瞿平. 围生期宫内生长迟缓胎鼠的发育及其肝胰岛素信号转导蛋白的表达[J]. 北京大学学报(医学版), 2008, 40(5): 538-542. |
| [6] | 崔玉英, 唐朝枢, 耿彬. 束缚应激大鼠血小板及血管内膜的L-精氨酸/一氧化氮合酶/一氧化氮通路下调[J]. 北京大学学报(医学版), 2006, 38(3): 231-235. |
| [7] | 韩颖, 秦炯, 卜定方, 杨志仙, 常杏芝, 杜军保. 反复热性惊厥过程中γ-氨基丁酸B受体对一氧化氮/一氧化氮合酶体系的调节作用[J]. 北京大学学报(医学版), 2006, 38(2): 132-134. |
| [8] | 崔玉英, 张立克, 伍期专, 蒋宏锋, 唐朝枢, 耿彬. 溶血磷脂酸激活大鼠血小板L-精氨酸/一氧化氮通路[J]. 北京大学学报(医学版), 2005, 37(6): 603-607. |
| [9] | 焦红梅, 谢鹏雁. 替加色罗抑制内脏高敏大鼠直肠扩张反应和脊髓神经元性一氧化氮合酶的表达[J]. 北京大学学报(医学版), 2004, 36(4): 394-398. |
| [10] | 李爱军, 房军, 朱秀安. N-Arg对RCS大鼠遗传性视网膜变性视细胞凋亡的影响[J]. 北京大学学报(医学版), 2004, 36(4): 353-356. |
| [11] | 董献红, 李菊香, 钟光珍, 任永生, 吴胜英, 唐朝枢. 来源于肾上腺髓质素原的不同肽段对大鼠主动脉一氧化氮生成的影响[J]. 北京大学学报(医学版), 2003, 35(2): 146-149. |
| [12] | 崔彩莲, 马勤耘, 吴鎏桢, 李雪, 魏娜, 韩济生. 2/100 Hz电针抑制吗啡戒断大鼠中枢神经元型一氧化氮合酶的表达[J]. 北京大学学报(医学版), 2002, 34(4): 321-325. |
| [13] | 余兵, 何权瀛, 高占成, 虞有智. 白三烯受体拮抗剂对哮喘小鼠的气道炎症及肺内一氧化氮合酶的影响[J]. 北京大学学报(医学版), 2002, 34(1): 52-56. |
| [14] | 饶煜, 库宝善. 褪黑激素对大鼠缺血/再灌注所致脑损伤的保护机制[J]. 北京大学学报(医学版), 2001, 33(2): 164-166. |
| [15] | 王俊梅, 宋明清, 刘斌. 诱生型一氧化氮合酶抑制剂氨基胍对鼠胚发育的影响[J]. 北京大学学报(医学版), 2001, 33(2): 153-156. |
|
||