北京大学学报(医学版) ›› 2022, Vol. 54 ›› Issue (5): 971-980. doi: 10.19723/j.issn.1671-167X.2022.05.026
阿替利珠单抗治疗中国晚期实体瘤患者的开放标签Ⅰ期临床试验
张力1,龚继芳2,潘宏铭3,白玉贤4,刘天舒5,程颖6,陈亚池7,黄佳莹8,许婷婷8,葛飞娇8,许婉玲9,施佳10,胡夕春11,*( ),沈琳2,*(
),沈琳2,*( )
)
- 1. 中山大学附属肿瘤医院肿瘤内科, 广州 510060
2. 北京大学肿瘤医院暨北京市肿瘤防治研究所消化肿瘤内科, 恶性肿瘤发病机制及转化研究教育部重点实验室, 北京 100142
3. 浙江大学医学院附属邵逸夫医院肿瘤内科, 杭州 310020
4. 哈尔滨医科大学附属肿瘤医院肿瘤内科, 哈尔滨 150081
5. 复旦大学附属中山医院肿瘤内科, 上海 200032
6. 吉林省肿瘤医院肿瘤内科, 长春 130012
7. Clinical Pharmacology, Genentech, Inc., South San Francisco, CA 94080, USA
8. 罗氏全球药品开发中国中心临床科学部, 上海 201203
9. 罗氏全球药品开发中国中心统计部, 上海 201203
10. 罗氏全球药品开发中国中心安全部, 上海 201203
11. 复旦大学附属肿瘤医院肿瘤内科, 上海 200032
Atezolizumab therapy in Chinese patients with locally advanced or metastatic solid tumors: An open-label, phase Ⅰ study
Li ZHANG1,Ji-fang GONG2,Hong-ming PAN3,Yu-xian BAI4,Tian-shu LIU5,Ying CHENG6,Ya-chi CHEN7,Jia-ying HUANG8,Ting-ting XU8,Fei-jiao GE8,Wan-ling HSU9,Jane SHI10,Xi-chun HU11,*( ),Lin SHEN2,*(
),Lin SHEN2,*( )
)
- 1. Department of Medical Oncology, Sun Yat-sen University Cancer Center, Guangzhou 510060, China
2. Key Laboratory of Carcinogenesis and Translational Research, Ministry of Education; Department of Gastrointestinal Oncology, Peking University Cancer Hospital & Institute, Beijing 100142, China
3. Department of Medical Oncology, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou 310020, China
4. Department of Medical Oncology, Harbin Medical University Cancer Hospital, Harbin 150081, China
5. Department of Medical Oncology, Zhongshan Hospital, Fudan University, Shanghai 200032, China
6. Department of Medical Oncology, Jilin Cancer Hospital, Changchun 130012, China
7. Clinical Pharmacology, Genentech, Inc., South San Francisco, CA 94080, USA
8. Oncology, Roche Product Development Shanghai, Shanghai, 201203, China
9. Department of Statistics, Roche Product Development Shanghai, Shanghai 201203, China
10. Portfolio Clinical Safety, Roche Product Development Shanghai, Shanghai 201203, China
11. Department of Medical Oncology, Fudan University Shanghai Cancer Center, Shanghai 200032, China
摘要:
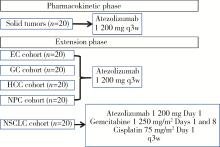
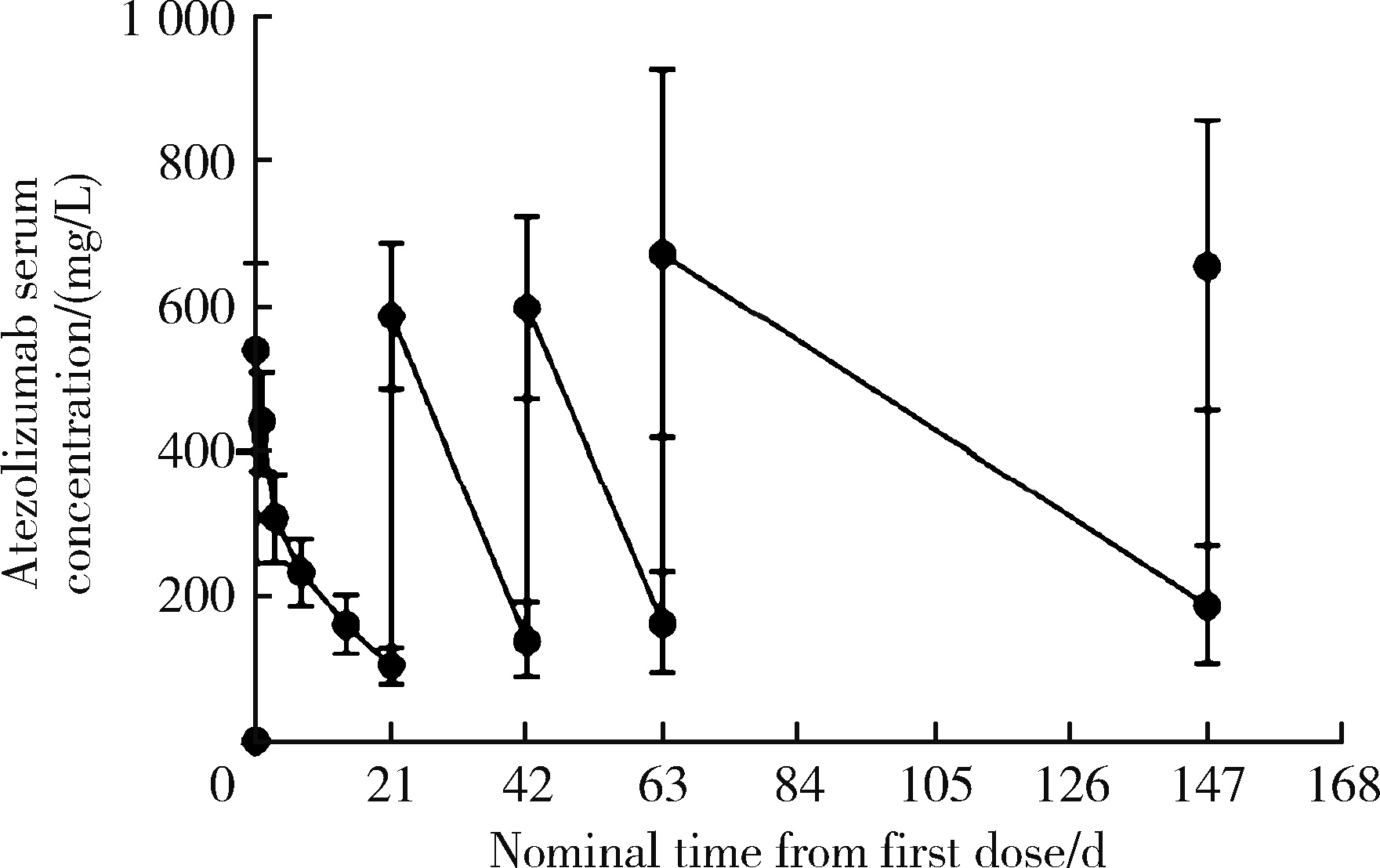
目的: 观察程序性细胞死亡配体1(programmed death-ligand 1,PD-L1)抑制剂阿替利珠单抗在中国高发实体瘤,包括食管癌(esophageal cancer,EC)、胃癌(gastric cancer,GC)、肝细胞癌(hepatocellular carcinoma,HCC)、鼻咽癌(nasopharyngeal cancer,NPC)和非小细胞肺癌(non-small cell lung cancer,NSCLC)患者中的药代动力学(pharmacokinetics,PK)、疗效和安全性数据。方法: 本研究为开放标签的Ⅰ期临床试验,于2016年8月4日至2019年4月15日在中国6个研究中心进行。入组患者年龄≥18岁,患有经组织学证实的无法治愈或转移性的实体瘤,且既往抗肿瘤治疗失败。PK阶段研究了阿替利珠单抗单药治疗的PK和安全性;扩展阶段研究了阿替利珠单抗单药治疗(入组EC、GC、HCC、NPC患者)和联合化疗(入组NSCLC患者)的安全性和有效性。结果: 共入组120例患者(PK阶段20例;扩展期每队列20例)。阿替利珠单抗单药组患者(n=100)中有42例(42.0%)为PD-L1阳性,9例(9.0%)为微卫星高度不稳定性。阿替利珠单抗的清除率为0.219 L/d,重复给药6~9周(2~3个周期)后达到稳态。EC、GC、HCC、NPC和NSCLC的客观缓解率(objective response rate,ORR)分别为10.0%、15.0%、10.0%、5.0%和40.0%。在PD-L1阳性的肿瘤患者中,阿替利珠单抗的ORR为11.9%,阿替利珠单抗联合吉西他滨和顺铂的ORR为46.2%。2例GC患者在假性进展后获得了持久的肿瘤缩小。阿替利珠单抗单药组最常见的治疗相关不良事件是疲劳、贫血、发热和白细胞计数减少,联合组最常见的治疗相关AE是贫血、白细胞计数减少和食欲下降。本试验没有发现新的安全信号。结论: 中国患者应用阿替利珠单抗的PK、疗效和安全性与之前研究中入组的全球患者的数据相似。
中图分类号:
- R730.51
| 1 | International Agency for Research on Cancer. China (Globocan 2020)[EB/OL]. (2021-03)[2021-05-15] https://gco.iarc.fr/today/data/factsheets/populations/160-china-fact-sheets.pdf. |
| 2 |
Arnold M , Soerjomataram I , Ferlay J , et al. Global incidence of oesophageal cancer by histological subtype in 2012[J]. Gut, 2015, 64 (3): 381- 387.
doi: 10.1136/gutjnl-2014-308124 |
| 3 | Salehiniya H , Mohammadian M , Mohammadian-Hafshejani A , et al. Nasopharyngeal cancer in the world: Epidemiology, incidence, mortality and risk factors[J]. World Cancer Res J, 2018, 5 (1): e1046. |
| 4 | Merck. Highlights of prescribing information. Keytruda® (Pembrolizumab)[EB/OL]. (2014)[2022-05-12]. https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf. |
| 5 |
Fuchs CS , Doi T , Jang RW , et al. Safety and efficacy of pembro-lizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial[J]. JAMA Oncol, 2018, 4 (5): e180013.
doi: 10.1001/jamaoncol.2018.0013 |
| 6 | Bristol Myers Squibb Company. Highlights of prescribing information. Opdivo (Nivolumab)[EB/OL]. (2014)[2022-05-03]. https://packageinserts.bms.com/pi/pi_opdivo.pdf. |
| 7 |
Ma BBY , Lim WT , Goh BC , et al. Antitumor activity of ni-volumab in recurrent and metastatic nasopharyngeal carcinoma: An international, multicenter study of the Mayo clinic phase 2 consortium (NCI-9742)[J]. J Clin Oncol, 2018, 36 (14): 1412- 1418.
doi: 10.1200/JCO.2017.77.0388 |
| 8 | NCCN Clinical Practice Guidelines in Oncology. Non-small cell lung cancer. Version 3.2022[EB/OL]. (2022-03-16)[2022-05]. http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. |
| 9 |
Herbst RS , Soria JC , Kowanetz M , et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients[J]. Nature, 2014, 515 (7528): 563- 567.
doi: 10.1038/nature14011 |
| 10 |
Weinstock C , Khozin S , Suzman D , et al. U.S. Food and Drug Administration approval summary: Atezolizumab for metastatic non-small cell lung cance[J]. Clin Cancer Res, 2017, 23 (16): 4534- 4539.
doi: 10.1158/1078-0432.CCR-17-0540 |
| 11 | Genentech. FDA approves Genentech's Tecentriq plus chemothe-rapy (abraxane and carboplatin) for the initial treatment of metastatic non-squamous non-small cell lung cancer[N/OL]. (2019-12-03)[2021-05-03] https://www.gene.com/media/press-releases/14827/2019-12-03/fda-approves-genentechs-tecentriq-plus-c. |
| 12 | F. Hoffmann-La Roche Ltd. European Commission approves Roche's new Tecentriq-based combination therapy as an initial treatment for most common form of advanced lung cancer[N/OL]. (2019-09-06)[2021-05-11] https://www.roche.com/media/releases/med-cor-2019-09-06.htm. |
| 13 | F. Hoffmann-La Roche Ltd. FDA approves Roche's Tecentriq in combination with Avastin for people with the most common form of liver cancer[N/OL]. (2020-06-02)[2021-05-15] https://www.roche.com/media/releases/med-cor-2020-06-02.htm. |
| 14 |
Finn RS , Qin S , Ikeda M , et al. Atezolizumab plus Bevacizumab in unresectable hepatocellular carcinoma[J]. N Engl J Med, 2020, 382 (20): 1894- 1905.
doi: 10.1056/NEJMoa1915745 |
| 15 | Xu ZY , Brown L , Pan GW , et al. Lifestyle, environmental pollution and lung cancer in cities of Liaoning in northeastern China[J]. Lung Cancer, 1996, 14 (Suppl 1): S149- S160. |
| 16 |
Grenade C , Phelps MA , Villalona-Calero MA . Race and ethnicity in cancer therapy: What have we learned?[J]. Clin Pharmacol Ther, 2014, 95 (4): 403- 412.
doi: 10.1038/clpt.2014.5 |
| 17 | Genentech. Highlights of prescribing information. Tecentriq®(atezolizumab)[EB/OL]. (2016)[2022-05-15]. https://www.gene.com/download/pdf/tecentriq_prescribing.pdf. |
| 18 |
West H , McCleod M , Hussein M , et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial[J]. Lancet Oncol, 2019, 20 (7): 924- 937.
doi: 10.1016/S1470-2045(19)30167-6 |
| 19 |
Lee MS , Ryoo BY , Hsu CH , et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): An open-label, multicentre, phase 1b study[J]. Lancet Oncol, 2020, 21 (6): 808- 820.
doi: 10.1016/S1470-2045(20)30156-X |
| 20 |
Zhang T , Xie J , Arai S , et al. The efficacy and safety of anti-PD-1/PD-L1 antibodies for treatment of advanced or refractory can-cers: A meta-analysis[J]. Oncotarget, 2016, 7 (45): 73068- 73079.
doi: 10.18632/oncotarget.12230 |
| 21 |
Le DT , Kavan P , Kim TW , et al. KEYNOTE-164: Pembrolizu-mab for patients with advanced microsatellite instability high (MSI-H) colorectal cancer[J]. J Clin Oncol, 2018, 36 (Suppl 15): 3514.
doi: 10.1200/jco.2018.36.15_suppl.3514 |
| 22 |
Colevas AD , Bahleda R , Braiteh F , et al. Safety and clinical activity of atezolizumab in head and neck cancer: Results from a phase Ⅰ trial[J]. Ann Oncol, 2018, 29 (11): 2247- 2253.
doi: 10.1093/annonc/mdy411 |
| 23 |
Schiller JH , Harrington D , Belani CP , et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer[J]. N Engl J Med, 2002, 346 (2): 92- 98.
doi: 10.1056/NEJMoa011954 |
| 24 | International Agency for Research on Cancer. All cancers (Globocan 2020)[EB/OL]. (2020-12)[2022-05-12] https://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sheet.pdf. |
| [1] | 李志存, 吴天俣, 梁磊, 范宇, 孟一森, 张骞. 穿刺活检单针阳性前列腺癌术后病理升级的危险因素分析及列线图模型构建[J]. 北京大学学报(医学版), 2024, 56(5): 896-901. |
| [2] | 刘家骏, 刘国康, 朱玉虎. 免疫相关性重症肺炎1例[J]. 北京大学学报(医学版), 2024, 56(5): 932-937. |
| [3] | 黄教悌,胡菁,韩博. 治疗相关神经内分泌前列腺癌机制研究与靶向治疗新进展[J]. 北京大学学报(医学版), 2024, 56(4): 557-561. |
| [4] | 田宇轩,阮明健,刘毅,李德润,吴静云,沈棋,范宇,金杰. 双参数MRI改良PI-RADS评分4分和5分病灶的最大径对临床有意义前列腺癌的预测效果[J]. 北京大学学报(医学版), 2024, 56(4): 567-574. |
| [5] | 姚凯烽,阮明健,李德润,田宇轩,陈宇珂,范宇,刘毅. 靶向穿刺联合区域系统穿刺对PI-RADS 4~5分患者的前列腺癌诊断效能[J]. 北京大学学报(医学版), 2024, 56(4): 575-581. |
| [6] | 欧俊永,倪坤明,马潞林,王国良,颜野,杨斌,李庚午,宋昊东,陆敏,叶剑飞,张树栋. 肌层浸润性膀胱癌合并中高危前列腺癌患者的预后因素[J]. 北京大学学报(医学版), 2024, 56(4): 582-588. |
| [7] | 王滨帅,邱敏,张前进,田茂锋,刘磊,王国良,陆敏,田晓军,张树栋. 6例肾尤文肉瘤伴静脉瘤栓的诊治[J]. 北京大学学报(医学版), 2024, 56(4): 636-639. |
| [8] | 虞乐,邓绍晖,张帆,颜野,叶剑飞,张树栋. 具有低度恶性潜能的多房囊性肾肿瘤的临床病理特征及预后[J]. 北京大学学报(医学版), 2024, 56(4): 661-666. |
| [9] | 舒帆,郝一昌,张展奕,邓绍晖,张洪宪,刘磊,王国良,田晓军,赵磊,马潞林,张树栋. 肾部分切除术治疗囊性肾癌的功能学和肿瘤学结果:单中心回顾性研究[J]. 北京大学学报(医学版), 2024, 56(4): 667-672. |
| [10] | 方杨毅,李强,黄志高,陆敏,洪锴,张树栋. 睾丸鞘膜高分化乳头状间皮肿瘤1例[J]. 北京大学学报(医学版), 2024, 56(4): 741-744. |
| [11] | 靖婷,江华,李婷,申倩倩,叶兰,曾银丹,梁文欣,冯罡,司徒文佑,张玉梅. 中国西部5城市中老年人血清25羟基维生素D与握力的相关性[J]. 北京大学学报(医学版), 2024, 56(3): 448-455. |
| [12] | 王清波,傅虹桥. 中国卫生筹资转型的主要特征与历史沿革[J]. 北京大学学报(医学版), 2024, 56(3): 462-470. |
| [13] | 柴晓东,孙子文,李海爽,朱靓怡,刘小旦,刘延涛,裴斐,常青. 髓母细胞瘤分子亚型中CD8+T淋巴细胞浸润的临床病理特点[J]. 北京大学学报(医学版), 2024, 56(3): 512-518. |
| [14] | 林国中,马长城,吴超,司雨,杨军. 微通道技术在颈椎管肿瘤微创切除术中的应用[J]. 北京大学学报(医学版), 2024, 56(2): 318-321. |
| [15] | 俞光岩. 儿童唾液腺疾病[J]. 北京大学学报(医学版), 2024, 56(1): 1-3. |
|
||