北京大学学报(医学版) ›› 2024, Vol. 56 ›› Issue (6): 972-979. doi: 10.19723/j.issn.1671-167X.2024.06.005
抗合成酶综合征重叠类风湿关节炎患者的免疫学特征
赵亮1, 史成龙1,2, 马柯3, 赵静1, 王潇4, 邢晓燕1, 莫万星1, 练益瑞1, 高超1, 李玉慧1,3,*( )
)
- 1. 北京大学人民医院风湿免疫科,北京 100044
2. 北京大学肿瘤医院暨北京市肿瘤防治研究所消化肿瘤内科,北京 100142
3. 北京大学人民医院青岛医院风湿免疫科,山东青岛 266111
4. 西双版纳傣族自治州人民医院血液风湿免疫科,云南西双版纳 666100
Immunological characteristics of patients with anti-synthetase syndrome overlap with rheumatoid arthritis
Liang ZHAO1, Chenglong SHI1,2, Ke MA3, Jing ZHAO1, Xiao WANG4, Xiaoyan XING1, Wanxing MO1, Yirui LIAN1, Chao GAO1, Yuhui LI1,3,*( )
)
- 1. Department of Rheumatology and Immunology, Peking University People' s Hospital, Beijing 100044, China
2. Department of Gastrointestinal Oncology, Peking University Cancer Hospital & Institute, Beijing 100142, China
3. Department of Rheumatology and Immunology, Peking University People' s Hospital, Qingdao, Qingdao 266111, Shandong, China
4. Department of Hematology and Rheumatology, the People' s Hospital of Xishuangbanna Dai Nationality Autonomous Prefecture, Xishuangbanna 666100, Yunnan, China
摘要:
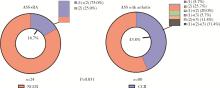
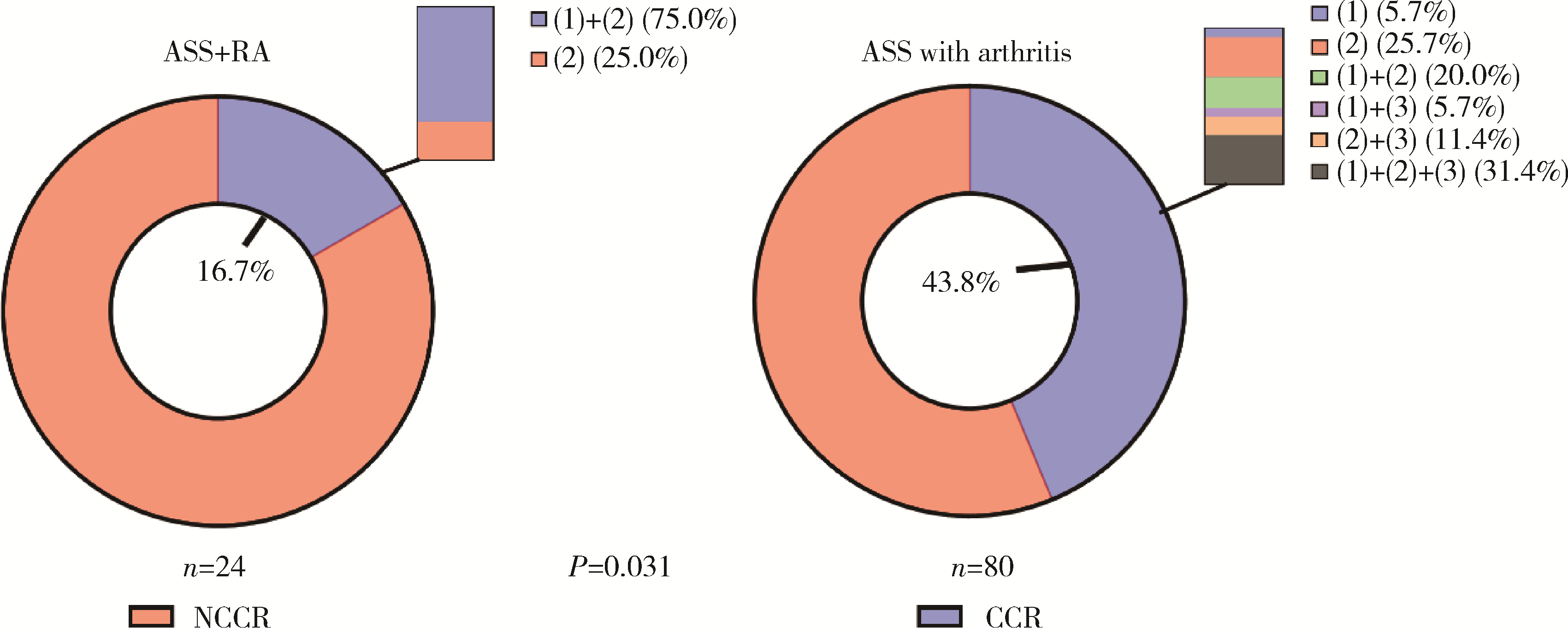
目的: 探究抗合成酶综合征(anti-synthetase syndrome, ASS)重叠类风湿关节炎(rheumatoid arthritis, RA)患者的免疫学特点。方法: 回顾性分析北京大学人民医院住院的104例ASS伴有关节炎患者的资料,包括人口学资料、临床表现(皮疹、肌肉损害等)、实验室检查结果、外周血淋巴细胞亚群和治疗用药,依据患者是否重叠RA进行分组并比较。结果: 104例ASS伴有关节炎的患者中,明确诊断RA的患者占23.1%(24/104例),ASS重叠RA组患者的快速进展型间质性肺炎(rapid progressive interstitial lung disease, RP-ILD)发生率(41.7% vs. 17.6%,P=0.032)、压痛关节数[10 (7, 14)个vs. 4 (0, 8)个,P<0.001]、肿胀关节数[4 (2, 8)个vs. 2 (0, 4)个,P=0.012]及骨侵蚀发生率(47.8% vs. 2.5%,P<0.001)均显著高于非RA组。实验室检查方面,血小板水平[(289.57±68.74)×103/μL vs. (247.94±77.04)×103/μL, P=0.022]、红细胞沉降率[43 (19, 59) mm/h vs. 18 (10, 44) mm/h, P=0.019]和C反应蛋白[19.20 (4.80, 55.36) mg/L vs. 5.68 (1.10, 14.96) mg/L, P=0.006] 在ASS重叠RA组的患者中显著升高。免疫学指标检查显示,与ASS合并关节炎的患者组相比,ASS重叠RA组患者的外周血CLA+Treg细胞[(11.12±4.10)% vs. (17.22±8.49)%,P=0.003]、B细胞[8.56% (4.80%, 11.90%) vs. 14.55% (8.75%, 20.29%),P=0.025]、自然杀伤(natural killer,NK)细胞[7.56% (4.65%, 13.20%) vs. 13.25% (7.46%, 19.25%),P=0.045] 的比例显著降低,Naïve Th细胞[(52.66±17.66)% vs. (40.76±14.96)%,P=0.033]的比例显著升高。治疗反应方面,ASS重叠RA组患者的完全临床应答率较低(16.7% vs. 43.8%, P=0.031)。结论: ASS重叠RA患者的肺部及关节受累更严重,治疗应答率低,对此类患者需早期识别并积极干预。
中图分类号:
- R593.2
| 1 | Marguerie C , Bunn CC , Beynon HL , et al. Polymyositis, pulmonary fibrosis and autoantibodies to aminoacyl-tRNA synthetase enzymes[J]. Q J Med, 1990, 77 (282): 1019- 1038. |
| 2 |
Aggarwal R , Cassidy E , Fertig N , et al. Patients with non-Jo-1 anti-tRNA-synthetase autoantibodies have worse survival than Jo-1 positive patients[J]. Ann Rheum Dis, 2014, 73 (1): 227- 232.
doi: 10.1136/annrheumdis-2012-201800 |
| 3 |
Hervier B , Devilliers H , Stanciu R , et al. Hierarchical cluster and survival analyses of antisynthetase syndrome: Phenotype and outcome are correlated with anti-tRNA synthetase antibody specificity[J]. Autoimmun Rev, 2012, 12 (2): 210- 217.
doi: 10.1016/j.autrev.2012.06.006 |
| 4 |
Labrador-Horrillo M , Martinez MA , Selva-O'Callaghan A , et al. Anti-cyclic citrullinated peptide and anti-keratin antibodies in patients with idiopathic inflammatory myopathy[J]. Rheumatology (Oxford), 2009, 48 (6): 676- 679.
doi: 10.1093/rheumatology/kep065 |
| 5 |
Cavagna L , Nuno L , Scirè CA , et al. Clinical spectrum time course in anti Jo-1 positive antisynthetase syndrome: Results from an international retrospective multicenter study[J]. Medicine (Baltimore), 2015, 94 (32): e1144.
doi: 10.1097/MD.0000000000001144 |
| 6 |
Lekieffre M , Gallay L , Landon-Cardinal O , et al. Joint and muscle inflammatory disease: A scoping review of the published evidence[J]. Semin Arthritis Rheum, 2023, 61, 152227.
doi: 10.1016/j.semarthrit.2023.152227 |
| 7 |
Marco JL , Collins BF . Clinical manifestations and treatment of antisynthetase syndrome[J]. Best Pract Res Clin Rheumatol, 2020, 34 (4): 101503.
doi: 10.1016/j.berh.2020.101503 |
| 8 |
Connors GR , Christopher-Stine L , Oddis CV , et al. Interstitial lung disease associated with the idiopathic inflammatory myopathies: What progress has been made in the past 35 years?[J]. Chest, 2010, 138 (6): 1464- 1474.
doi: 10.1378/chest.10-0180 |
| 9 |
Aletaha D , Neogi T , Silman AJ , et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative[J]. Ann Rheum Dis, 2010, 69 (9): 1580- 1588.
doi: 10.1136/ard.2010.138461 |
| 10 |
Oddis CV , Rider LG , Reed AM , et al. International consensus guidelines for trials of therapies in the idiopathic inflammatory myopathies[J]. Arthritis Rheum, 2005, 52 (9): 2607- 2615.
doi: 10.1002/art.21291 |
| 11 |
Aggarwal R , Rider LG , Ruperto N , et al. 2016 American College of Rheumatology/European League Against Rheumatism criteria for minimal, moderate, and major clinical response in adult dermatomyositis and polymyositis: An International Myositis Assessment and Clinical Studies Group/Paediatric Rheumatology International Trials Organisation collaborative initiative[J]. Ann Rheum Dis, 2017, 76 (5): 792- 801.
doi: 10.1136/annrheumdis-2017-211400 |
| 12 | 赖展鸿, 李嘉辰, 贠泽霖, 等. 特发性炎性肌病完全临床应答相关因素的单中心真实世界研究[J]. 北京大学学报(医学版), 2024, 56 (2): 284- 292. |
| 13 | Li Y , Gao X , Li Y , et al. Predictors and mortality of rapidly progressive interstitial lung disease in patients with idiopathic inflammatory myopathy: A series of 474 patients[J]. Front Med (Lausanne), 2020, 7, 363. |
| 14 |
Jablonski R , Bhorade S , Strek ME , et al. Recognition and ma-nagement of myositis-associated rapidly progressive interstitial lung disease[J]. Chest, 2020, 158 (1): 252- 263.
doi: 10.1016/j.chest.2020.01.033 |
| 15 |
Go DJ , Park JK , Kang EH , et al. Survival benefit associated with early cyclosporine treatment for dermatomyositis-associated interstitial lung disease[J]. Rheumatol Int, 2016, 36 (1): 125- 131.
doi: 10.1007/s00296-015-3328-8 |
| 16 |
Xu Y , Yang CS , Li YJ , et al. Predictive factors of rapidly progressive-interstitial lung disease in patients with clinically amyopathic dermatomyositis[J]. Clin Rheumatol, 2016, 35 (1): 113- 116.
doi: 10.1007/s10067-015-3139-z |
| 17 | Milovanovic M , Nilsson E , Järemo P . Relationships between platelets and inflammatory markers in rheumatoid arthritis[J]. Clin Chim Acta, 2004, 343 (1/2): 237- 240. |
| 18 |
Yazici S , Yazici M , Erer B , et al. The platelet indices in patients with rheumatoid arthritis: Mean platelet volume reflects disease activity[J]. Platelets, 2010, 21 (2): 122- 125.
doi: 10.3109/09537100903474373 |
| 19 |
Liu Y , Jiang H , Kang T , et al. Platelets-related signature based diagnostic model in rheumatoid arthritis using WGCNA and machine learning[J]. Front Immunol, 2023, 14, 1204652.
doi: 10.3389/fimmu.2023.1204652 |
| 20 | Rosengren S , Corr M , Boyle DL . Platelet-derived growth factor and transforming growth factor beta synergistically potentiate inflammatory mediator synthesis by fibroblast-like synoviocytes[J]. Arthritis Res Ther, 2010, 12 (2): 1- 11. |
| 21 |
Lefèvre S , Schwarz M , Meier FMP , et al. Disease-specific effects of matrix and growth factors on adhesion and migration of rheumatoid synovial fibroblasts[J]. J Immunol, 2017, 198 (12): 4588- 4595.
doi: 10.4049/jimmunol.1600989 |
| 22 |
Brown AJ , Sepuru KM , Sawant KV , et al. Platelet-derived chemokine CXCL7 dimer preferentially exists in the glycosaminoglycan-bound form: Implications for neutrophil-platelet crosstalk[J]. Front Immunol, 2017, 8, 1248.
doi: 10.3389/fimmu.2017.01248 |
| 23 |
Gáspár K , Baráth S , Nagy G , et al. Regulatory T-cell subsets with acquired functional impairment: Important indicators of disease severity in atopic dermatitis[J]. Acta Derm Venereol, 2015, 95 (2): 151- 155.
doi: 10.2340/00015555-1882 |
| 24 |
Li J , Sun F , Zhu D , et al. Deficiency of peripheral CLA(+) Tregs and clinical relevance in Behcet' s syndrome[J]. Arthritis Res Ther, 2024, 26 (1): 76.
doi: 10.1186/s13075-024-03306-9 |
| 25 |
Chalan P , Bijzet J , Kroesen BJ , et al. Altered natural killer cell subsets in seropositive arthralgia and early rheumatoid arthritis are associated with autoantibody status[J]. J Rheumatol, 2016, 43 (6): 1008- 1016.
doi: 10.3899/jrheum.150644 |
| 26 |
Aggarwal A , Sharma A , Bhatnagar A . Role of cytolytic impairment of natural killer and natural killer T-cell populations in rheumatoid arthritis[J]. Clin Rheumatol, 2014, 33 (8): 1067- 1078.
doi: 10.1007/s10067-014-2641-z |
| 27 |
Söderström K , Stein E , Colmenero P , et al. Natural killer cells trigger osteoclastogenesis and bone destruction in arthritis[J]. Proc Natl Acad Sci USA, 2010, 107 (29): 13028- 13033.
doi: 10.1073/pnas.1000546107 |
| 28 |
Romas E , Gillespie MT , Martin TJ . Involvement of receptor activator of NF kappa B ligand and tumor necrosis factor-alpha in bone destruction in rheumatoid arthritis[J]. Bone, 2002, 30 (2): 340- 346.
doi: 10.1016/S8756-3282(01)00682-2 |
| 29 |
Leipe J , Grunke M , Dechant C , et al. Role of Th17 cells in human autoimmune arthritis[J]. Arthritis Rheum, 2010, 62 (10): 2876- 2885.
doi: 10.1002/art.27622 |
| 30 |
Takayanagi H , Ogasawara K , Hida S , et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma[J]. Nature, 2000, 408 (6812): 600- 605.
doi: 10.1038/35046102 |
| 31 |
Harrington LE , Hatton RD , Mangan PR , et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages[J]. Nat Immunol, 2005, 6 (11): 1123- 1132.
doi: 10.1038/ni1254 |
| 32 |
Lundberg IE , Fujimoto M , Vencovsky J , et al. Idiopathic inflammatory myopathies[J]. Nat Rev Dis Primers, 2021, 7 (1): 86.
doi: 10.1038/s41572-021-00321-x |
| 33 |
Witt LJ , Curran JJ , Strek ME . The diagnosis and treatment of antisynthetase syndrome[J]. Clin Pulm Med, 2016, 23 (5): 218- 226.
doi: 10.1097/CPM.0000000000000171 |
| 34 |
Baumann Benvenuti F , Dudler J . Long-lasting improvement of refractory antisynthetase syndrome with tocilizumab: A report of two cases[J]. RMD Open, 2023, 9 (4): e003599.
doi: 10.1136/rmdopen-2023-003599 |
| 35 |
Narváez J , Cañadillas E , Castellví I , et al. Rituximab in the treatment of progressive interstitial lung disease associated with the antisynthetase syndrome[J]. Arthritis Res Ther, 2024, 26 (1): 122.
doi: 10.1186/s13075-024-03353-2 |
| 36 |
Smolen JS , Beaulieu A , Rubbert-Roth A , et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): A double-blind, placebo-controlled, randomised trial[J]. Lancet, 2008, 371 (9617): 987- 997.
doi: 10.1016/S0140-6736(08)60453-5 |
| [1] | 贾霈雯, 杨迎, 邹耀威, 欧阳志明, 林建子, 马剑达, 杨葵敏, 戴冽. 类风湿关节炎患者低肌肉量综合征的临床特征及其对躯体功能的影响[J]. 北京大学学报(医学版), 2024, 56(6): 1009-1016. |
| [2] | 马豆豆, 卢哲敏, 郭倩, 朱莎, 古今, 丁艳, 石连杰. 小剂量利妥昔单抗成功治疗类风湿关节炎合并重症肌无力1例[J]. 北京大学学报(医学版), 2024, 56(6): 1110-1114. |
| [3] | 闫蕊, 柯丹, 张妍, 李丽, 苏焕然, 陈伟, 孙明霞, 刘晓敏, 罗靓. 血清趋化因子CXCL-10和涎液化糖链抗原6水平在类风湿关节炎合并肺间质病变患者中的诊断和病情评估价值[J]. 北京大学学报(医学版), 2024, 56(6): 956-962. |
| [4] | 朱玉静, 王磊, 吕成银, 谈文峰, 张缪佳. 抗EJ抗体阳性抗合成酶综合征相关间质性肺疾病复发的临床特征分析[J]. 北京大学学报(医学版), 2024, 56(6): 980-986. |
| [5] | 韩艺钧, 陈小莉, 李常虹, 赵金霞. 甲氨蝶呤在类风湿关节炎患者中的应用现状[J]. 北京大学学报(医学版), 2024, 56(6): 994-1000. |
| [6] | 刘东武, 陈杰, 高明利, 于静. 类风湿关节炎伴发淋巴结Castleman样病理改变1例[J]. 北京大学学报(医学版), 2024, 56(5): 928-931. |
| [7] | 黄会娜,赵静,赵祥格,白自然,李霞,王冠. 乳酸对类风湿关节炎患者外周血CD4+T细胞亚群的调控作用[J]. 北京大学学报(医学版), 2024, 56(3): 519-525. |
| [8] | 汤晓菲,李永红,丁秋玲,孙卓,张阳,王育梅,田美伊,刘坚. 类风湿关节炎患者下肢深静脉血栓发病率及危险因素[J]. 北京大学学报(医学版), 2024, 56(2): 279-283. |
| [9] | 邹雪,白小娟,张丽卿. 艾拉莫德联合托法替布治疗难治性中重度类风湿关节炎的疗效[J]. 北京大学学报(医学版), 2023, 55(6): 1013-1021. |
| [10] | 李嘉辰,赖展鸿,邵苗,金月波,高小娟,张科,侯儆,张燕英,栗占国,李玉慧. 抗Jo-1抗体在特发性炎性肌病临床分层及疾病谱中的意义[J]. 北京大学学报(医学版), 2023, 55(6): 958-965. |
| [11] | 吴琦,蔡月明,何娟,黄文蒂,王庆文. 血脂异常与类风湿关节炎肺间质病变的相关性分析[J]. 北京大学学报(医学版), 2023, 55(6): 982-992. |
| [12] | 张警丰,金银姬,魏慧,姚中强,赵金霞. 体重指数与类风湿关节炎临床特征的相关性分析[J]. 北京大学学报(医学版), 2023, 55(6): 993-999. |
| [13] | 金银姬,孙琳,赵金霞,刘湘源. 血清IgA型抗鼠科肉瘤病毒癌基因同源物B1抗体在类风湿关节炎中的意义[J]. 北京大学学报(医学版), 2023, 55(4): 631-635. |
| [14] | 蔡文心,李仕成,刘一鸣,梁如玉,李静,郭建萍,胡凡磊,孙晓麟,李春,刘栩,叶华,邓立宗,李茹,栗占国. 类风湿关节炎临床分层及其特征的横断面研究[J]. 北京大学学报(医学版), 2022, 54(6): 1068-1073. |
| [15] | 程昉,杨邵英,房星星,王璇,赵福涛. CCL28-CCR10通路在类风湿关节炎单核细胞迁移中的作用[J]. 北京大学学报(医学版), 2022, 54(6): 1074-1078. |
|
||