北京大学学报(医学版) ›› 2024, Vol. 56 ›› Issue (6): 980-986. doi: 10.19723/j.issn.1671-167X.2024.06.006
抗EJ抗体阳性抗合成酶综合征相关间质性肺疾病复发的临床特征分析
- 南京医科大学第一附属医院风湿免疫科,南京 210029
Analysis of clinical features of ruccrent interstitial lung disease in patients with anti-EJ positive antisynthetase syndrome
Yujing ZHU, Lei WANG, Chengyin LYU, Wenfeng TAN, Miaojia ZHANG*( )
)
- Department of Rheumatology and Immunology, the First Affiliated Hospital of Nanjing Medical University, Nanjing 210029, China
摘要:
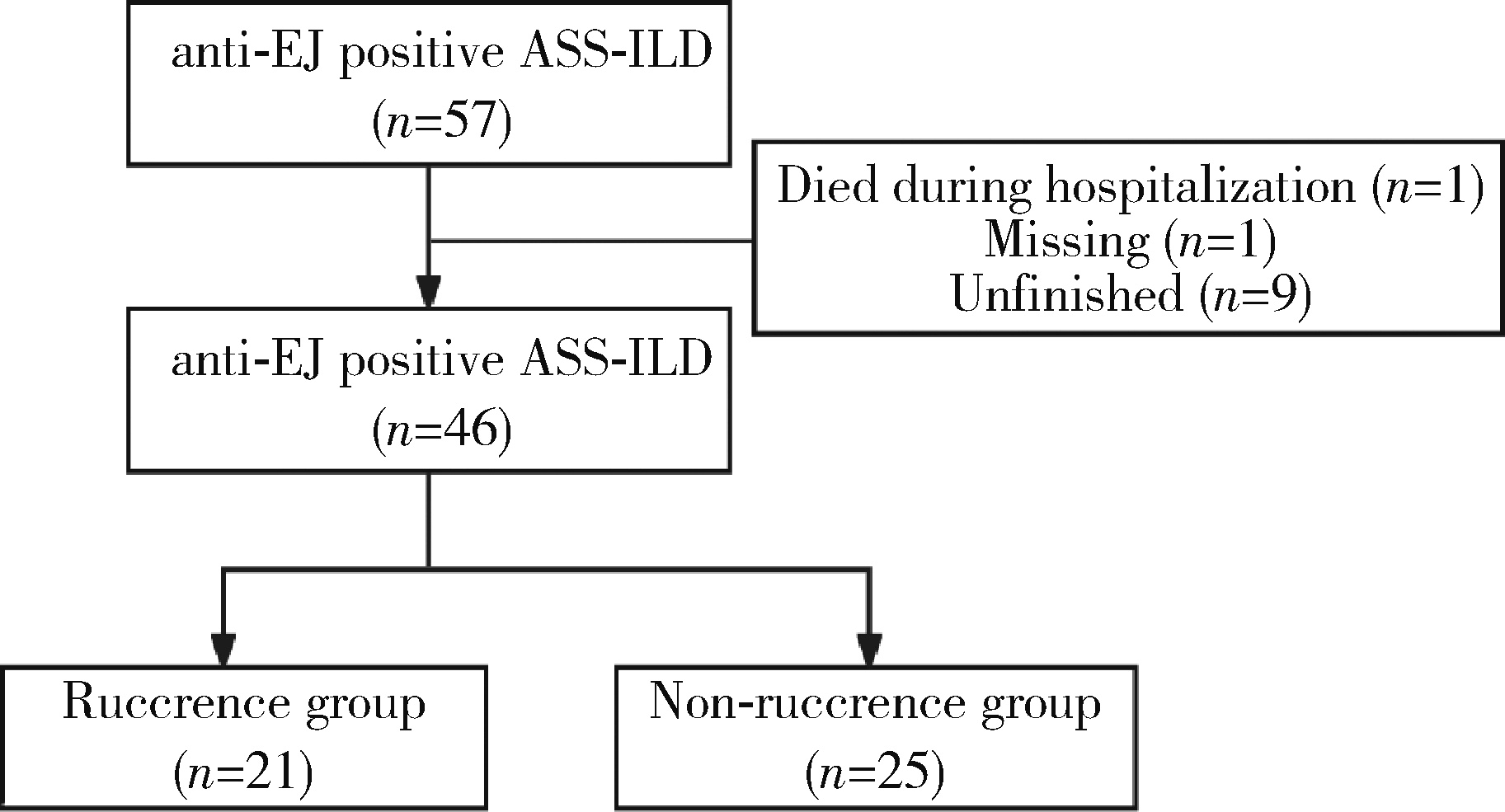
目的: 总结57例抗EJ (anti-glycyl tRNA synthetase) 抗体阳性抗合成酶综合征(antisynthetase syndrome, ASS) 相关间质性肺疾病(interstitial lung disease, ILD) 患者的临床特征,并分析ILD复发的相关因素。方法: 选择2020年1月1日至2024年6月30日在南京医科大学第一附属医院(江苏省人民医院)风湿免疫科就诊的57例抗EJ抗体阳性ASS患者的病例资料进行回顾性分析,收集的数据包括患者的一般情况、临床表现、实验室检查结果和胸部CT影像,以及肺功能检查结果[包括用力肺活量(forced vital capacity, FVC)、第1秒用力呼气容积(forced expiratory volume in the first second, FEV1) 和一氧化碳弥散量(diffusion lung carbon monoxide, DLCO)],并进一步分析ILD复发的临床特征。结果: 所有57例抗EJ抗体阳性ASS患者均合并ILD,平均发病年龄为(58.18±10.27)岁,平均病程为3.00 (2.00, 16.00)个月。患者中70. 18%为女性,87.72%有咳嗽症状,70. 18%有咳痰症状,89.47%出现呼吸困难,14.04%发生呼吸衰竭。肺功能结果显示,FVC占正常预计值的百分比(FVC%)、FEV1占正常预计值的百分比(FEV1%) 和DLCO占正常预计值的百分比(DLCO%) 分别为59.36±21.41、58.34±19.46和58.17±27.95,氧合指数为(363.24±99.42) mmHg。胸部CT影像中,非特异性间质性肺炎(nonspecific interstitial pneumonia, NSIP) 为最常见的影像学表现类型。在46例完成12个月以上随访的患者中,21例(45.65%) 出现了ILD复发。复发组的平均发病年龄为(61.38±8.63)岁,而非复发组为(55.28±11.85)岁,差异无统计学意义(P=0.056)。进一步分析发现,复发组的红细胞沉降率(erythrocyte sedimentation rate, ESR) 水平显著高于非复发组[(50.48±29.64) mm/h vs. 30.28±23.97) mm/h, P=0.025],血清IgM (immune globulin M, IgM) 水平也明显高于非复发组(P=0.042)。此外,复发组的CD8 + T细胞百分比显著高于非复发组(25.48±11.81 vs. 18.59± 8.53, P=0.027)。尽管复发组患者在基线时的年龄偏大,且ESR、IgM和CD8 + T细胞百分比在两组间差异有统计学意义,但多因素二元Logistics回归分析显示,这些指标均未被证实为ILD复发的独立危险因素。结论: ILD是抗EJ抗体阳性ASS患者的主要临床表现,并且常伴有显著的肺功能损伤。尽管糖皮质激素和免疫抑制剂的联合治疗对大多数患者有效,但ILD的复发率依然较高。基线ESR水平增快、IgM升高及CD8+ T细胞百分比增加的患者更容易出现复发。
中图分类号:
- R563
| 1 |
Aggarwal R , Cassidy E , Fertig N , et al. Patients with non-Jo-1 anti-tRNA synthetase autoantibodies have worse survival than Jo-1 positive patients[J]. Ann Rheum Dis, 2014, 73 (1): 227- 232.
doi: 10.1136/annrheumdis-2012-201800 |
| 2 | Sreevilasan SK , Devarasetti P , Narahari NK , et al. Clinical profile and treatment outcomes in antisynthetase syndrome: A tertiary centre experience[J]. Rheumatol Adv Pract, 2021, 5 (Suppl 2): ii10- ii18. |
| 3 |
Hamaguchi Y , Fujimoto M , Matsushita T , et al. Common and distinct clinical features in adult patients with anti-aminoacyl-tRNA synthetase antibodies: Heterogeneity within the syndrome[J]. PLoS One, 2013, 8 (4): e60442.
doi: 10.1371/journal.pone.0060442 |
| 4 |
Teel A , Lu J , Park J , et al. The role of myositis-specific autoantibodies and the management of interstitial lung disease in idiopathic inflammatory myopathies: A systematic review[J]. Semin Arthritis Rheum, 2022, 57, 152088.
doi: 10.1016/j.semarthrit.2022.152088 |
| 5 |
Zhang Y , Ge Y , Yang H , et al. Clinical features and outcomes of the patients with anti-glycyl tRNA synthetase syndrome[J]. Clin Rheumatol, 2020, 39 (8): 2417- 2424.
doi: 10.1007/s10067-020-04979-8 |
| 6 |
Watanabe K , Handa T , Tanizawa K , et al. Detection of antisynthetase syndrome in patients with idiopathic interstitial pneumonias[J]. Respir Med, 2011, 105 (8): 1238- 1247.
doi: 10.1016/j.rmed.2011.03.022 |
| 7 |
Sasano H , Hagiwara E , Kitamura H , et al. Long-term clinical course of anti-glycyl tRNA synthetase (anti-EJ) antibody-related interstitial lung disease pathologically proven by surgical lung biopsy[J]. BMC Pulm Med, 2016, 16 (1): 168.
doi: 10.1186/s12890-016-0325-y |
| 8 |
Hozumi H , Fujisawa T , Nakashima R , et al. Efficacy of glucocorticoids and calcineurin inhibitors for anti-aminoacyl-trna synthetase antibody-positive polymyositis/dermatomyositis-associated interstitial lung disease: A propensity score-matched analysis[J]. J Rheumatol, 2019, 46 (5): 509- 517.
doi: 10.3899/jrheum.180778 |
| 9 | Yorishima Y , Tominaga M , Fujimoto K , et al. Combination of prednisolone and calcineurin inhibitors prevents lung function decline in patients with anti-aminoacyl-tRNA synthetase antibody-positive polymyositis/dermatomyositis[J]. Kurume Med J, 2023, 69 (1/2): 19- 30. |
| 10 |
Martínez-García EA , Lujano-Benítez AV , Gercía-De La Torre Ⅰ , et al. Good response to mycophenolate mofetil on treatment of interstitial lung disease in polymyositis associated with antisynthetase syndrome positive for anti-EJ and anti-Ro52 antibodies[J]. Clin Rheumatol, 2020, 39 (9): 2837- 2839.
doi: 10.1007/s10067-020-05075-7 |
| 11 |
Langlois V , Gillibert A , Uzunhan Y , et al. Rituximab and cyclophosphamide in antisynthetase syndrome-related interstitial lung disease: An observational retrospective study[J]. J Rheumatol, 2020, 47 (11): 1678- 1686.
doi: 10.3899/jrheum.190505 |
| 12 |
Liu Y , Liu X , Xie M , et al. Clinical characteristics of patients with anti-EJ antisynthetase syndrome associated interstitial lung disease and literature review[J]. Respir Med, 2020, 165, 105920.
doi: 10.1016/j.rmed.2020.105920 |
| 13 |
Connors GR , Christopher-Stine L , Oddis CV , et al. Interstitial lung disease associated with the idiopathic inflammatory myopathies: What progress has been made in thepast 35 years[J]. Chest, 2010, 138 (6): 1464- 1474.
doi: 10.1378/chest.10-0180 |
| 14 |
Bohan A , Peter JB . Polymyositis and dermatomyositis (first of two parts)[J]. N Engl J Med, 1975, 292 (7): 344- 347.
doi: 10.1056/NEJM197502132920706 |
| 15 |
Raghu G , Remy-Jardin M , Richeldi L , et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: An official ATS/ERS/JRS /ALAT clinical practice guideline[J]. Am J Respir Crit Care Med, 2022, 205 (9): e18- e47.
doi: 10.1164/rccm.202202-0399ST |
| 16 |
Targoff IN . Autoantibodies to aminoacyl-transfer RNA synthetases for isoleucine and glycine. Two additional synthetases are antigenic in myositis[J]. J Immunol, 1990, 144 (5): 1737- 1743.
doi: 10.4049/jimmunol.144.5.1737 |
| 17 | Tang HS, Tang IYK, Ho RTC, et al. Clinical heterogeneity and prognostic factors of anti-synthetase syndrome: A multi-centered retrospective cohort study[J]. Rheumatology (Oxford), 2023 (2023-12-14)[2024-09-01]. https://Doi.org/10.1093/rheumatology/kead671. |
| 18 |
García-Bravo L , Calle-Rubio M , Fernández-Arquero M , et al. Association of anti-SARS-COV-2 vaccine with increased incidence of myositis-related anti-RNA-synthetases auto-antibodies[J]. J Transl Autoimmun, 2022, 5, 100160.
doi: 10.1016/j.jtauto.2022.100160 |
| 19 | Irie Y , Wakabayashi H , Matuzawa Y , et al. A case of anti-synthetase syndrome with anti-glycyl tRNA synthetases antibody de-veloped after COVID-19[J]. Cureus, 2024, 16 (4): e58004. |
| 20 |
Shimizu H , Matsumoto H , Sasajima T , et al. New-onset dermatomyositis following COVID-19: A case report[J]. Front Immunol, 2022, 13, 1002329.
doi: 10.3389/fimmu.2022.1002329 |
| 21 | Peña C , Kalara N , Velagapudi P . A case of antisynthetase syndrome in the setting of SARS-Cov-2 infection[J]. Cureus, 2023, 15 (6): e40588. |
| 22 |
Elsayed M , Abdelgabar A , Karmani J , et al. A case of antisynthetase syndrome initially presented with interstitial lung disease mimicking COVID-19[J]. J Med Cases, 2023, 14 (1): 25- 30.
doi: 10.14740/jmc4031 |
| 23 | Tranah E , MacBrayne A , Bhadauria N , et al. A case of antisynthetase syndrome presenting solely with life-threatening interstitial lung disease[J]. Clin Med (Lond), 2023, 23 (1): 85- 87. |
| 24 | 周云, 吕成银, 尤含笑, 等. 不同抗体亚型抗合成酶综合征并发间质性肺疾病临床特征分析[J]. 中华风湿病学杂志, 2024, 28 (8): 538- 544. |
| [1] | 李钰锴, 王红彦, 罗靓, 李云, 李春. 抗磷脂抗体在白塞病合并血栓中的临床意义[J]. 北京大学学报(医学版), 2024, 56(6): 1036-1040. |
| [2] | 李春, 胡晓丹. 关注血栓性抗磷脂综合征的长期抗凝[J]. 北京大学学报(医学版), 2024, 56(6): 947-949. |
| [3] | 赵亮, 史成龙, 马柯, 赵静, 王潇, 邢晓燕, 莫万星, 练益瑞, 高超, 李玉慧. 抗合成酶综合征重叠类风湿关节炎患者的免疫学特征[J]. 北京大学学报(医学版), 2024, 56(6): 972-979. |
| [4] | 李雨清,王飚,乔鹏,王玮,关星. 经耻骨后尿道中段悬吊带术治疗女性复发性压力性尿失禁的中长期疗效[J]. 北京大学学报(医学版), 2024, 56(4): 600-604. |
| [5] | 李文根,古晓东,翁锐强,刘苏东,陈超. 血浆外泌体miR-34-5p和miR-142-3p在系统性硬化症中的表达及临床意义[J]. 北京大学学报(医学版), 2023, 55(6): 1022-1027. |
| [6] | 姚中强,李常虹,李欣艺,郭苇,翟佳羽,刘蕊,魏慧,穆荣. 抗磷脂酰丝氨酸/凝血酶原抗体与不明原因复发性流产的相关性分析[J]. 北京大学学报(医学版), 2023, 55(6): 1058-1061. |
| [7] | 李嘉辰,赖展鸿,邵苗,金月波,高小娟,张科,侯儆,张燕英,栗占国,李玉慧. 抗Jo-1抗体在特发性炎性肌病临床分层及疾病谱中的意义[J]. 北京大学学报(医学版), 2023, 55(6): 958-965. |
| [8] | 陈素华,杨军,陈新,杨辰龙,孙建军,林国中,于涛,杨欣,韩芸峰,吴超,司雨,马凯明. 大型、巨大型上矢状窦中后1/3侵犯颅外复发脑膜瘤的手术治疗[J]. 北京大学学报(医学版), 2022, 54(5): 1006-1012. |
| [9] | 王跃,张爽,张虹,梁丽,徐玲,程元甲,段学宁,刘荫华,李挺. 激素受体阳性/人表皮生长因子受体2阴性乳腺癌临床病理特征及预后[J]. 北京大学学报(医学版), 2022, 54(5): 853-862. |
| [10] | 森本智惠子,王益勤,周蓉,王建六. 子宫内膜非典型增生及子宫内膜癌患者保留生育功能治疗的临床研究[J]. 北京大学学报(医学版), 2022, 54(5): 936-942. |
| [11] | 罗澜,邢晓燕,肖云抒,陈珂彦,朱冯赟智,张学武,李玉慧. 抗合成酶综合征合并心脏受累患者的临床及免疫学特征[J]. 北京大学学报(医学版), 2021, 53(6): 1078-1082. |
| [12] | 刘磊,秦艳春,王国良,张树栋,侯小飞,马潞林. 嗜铬细胞瘤和副神经节瘤二次手术策略[J]. 北京大学学报(医学版), 2021, 53(4): 793-797. |
| [13] | 夏芳芳,鲁芙爱,吕慧敏,杨国安,刘媛. 系统性红斑狼疮伴间质性肺炎的临床特点及相关因素分析[J]. 北京大学学报(医学版), 2021, 53(2): 266-272. |
| [14] | 于焕斌,伍文杰,吕晓鸣,石妍,郑磊,张建国. 125I粒子近距离治疗外放疗后复发唾液腺癌[J]. 北京大学学报(医学版), 2020, 52(5): 919-923. |
| [15] | 熊盛炜,王杰,朱伟杰,程嗣达,张雷,李学松,周利群. 二次肾盂成形术在复发性肾盂输尿管连接部梗阻中的研究进展[J]. 北京大学学报(医学版), 2020, 52(4): 794-798. |
|
||