北京大学学报(医学版) ›› 2025, Vol. 57 ›› Issue (5): 884-894. doi: 10.19723/j.issn.1671-167X.2025.05.012
HDAC2介导H3K27ac修饰促进肝癌细胞的增殖和迁移
唐少海1, 杨宝明1, 李建坤1, 赵丽丽2, 王仪凡3, 王顺祥1,*( )
)
- 1. 河北医科大学第四医院肝胆外科, 石家庄 050000
2. 河北医科大学药学院, 石家庄 050000
3. 河北医科大学第四医院结直肠外科, 石家庄 050000
HDAC2-mediated H3K27 acetylation promotes the proliferation and migration of hepatocellular carcinoma cells
Shaohai TANG1, Baoming YANG1, Jiankun LI1, Lili ZHAO2, Yifan WANG3, Shunxiang WANG1,*( )
)
- 1. Department of Hepatobiliary Surgery, the Fourth Hospital of Hebei Medical University, Shijiazhuang 050000, China
2. College of Pharmacy, Hebei Medical University, Shijiazhuang 050000, China;
3. Department of Colorectal Surgery, the Fourth Hospital of Hebei Medical University, Shijiazhuang 050000, China
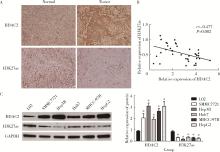
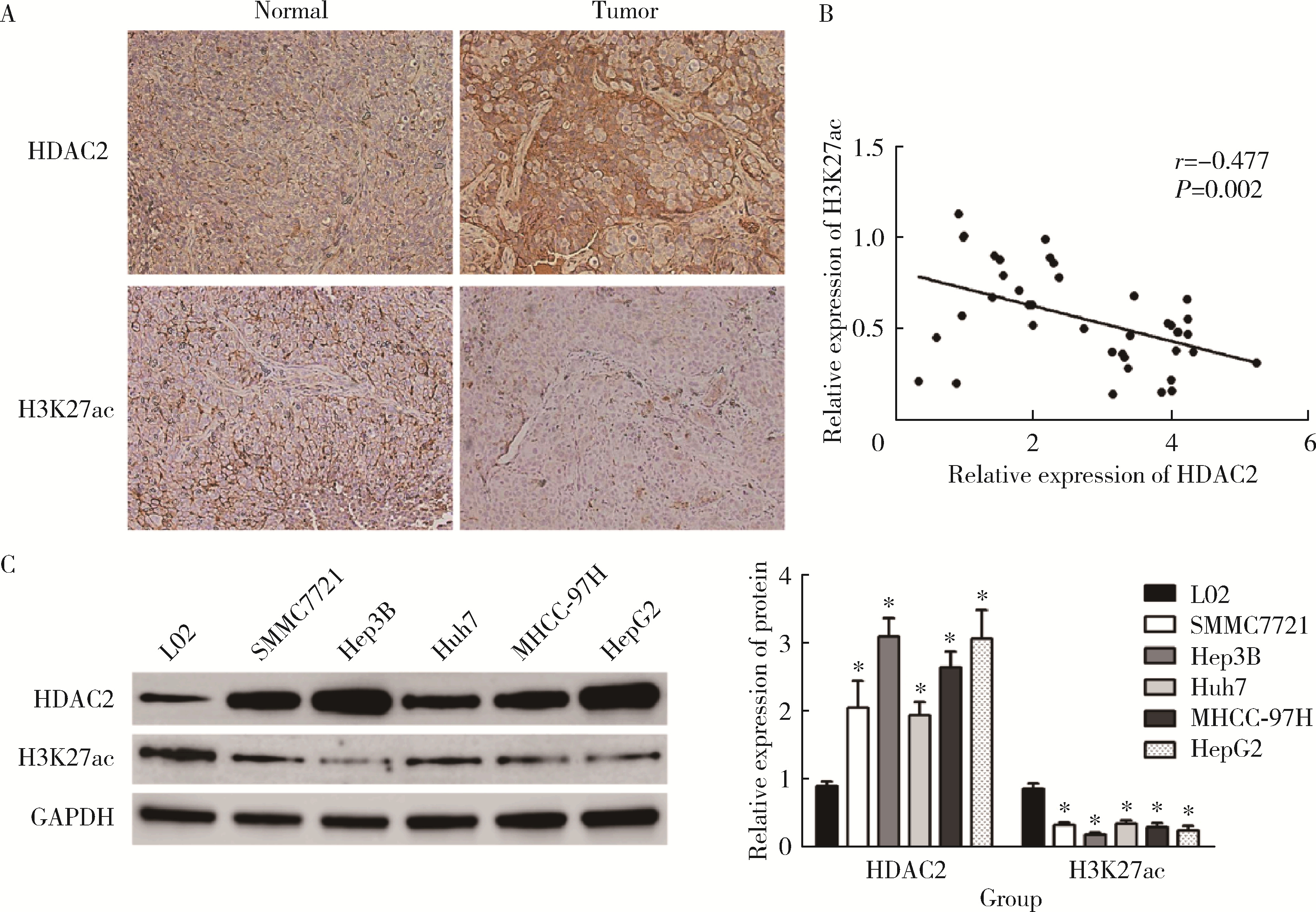
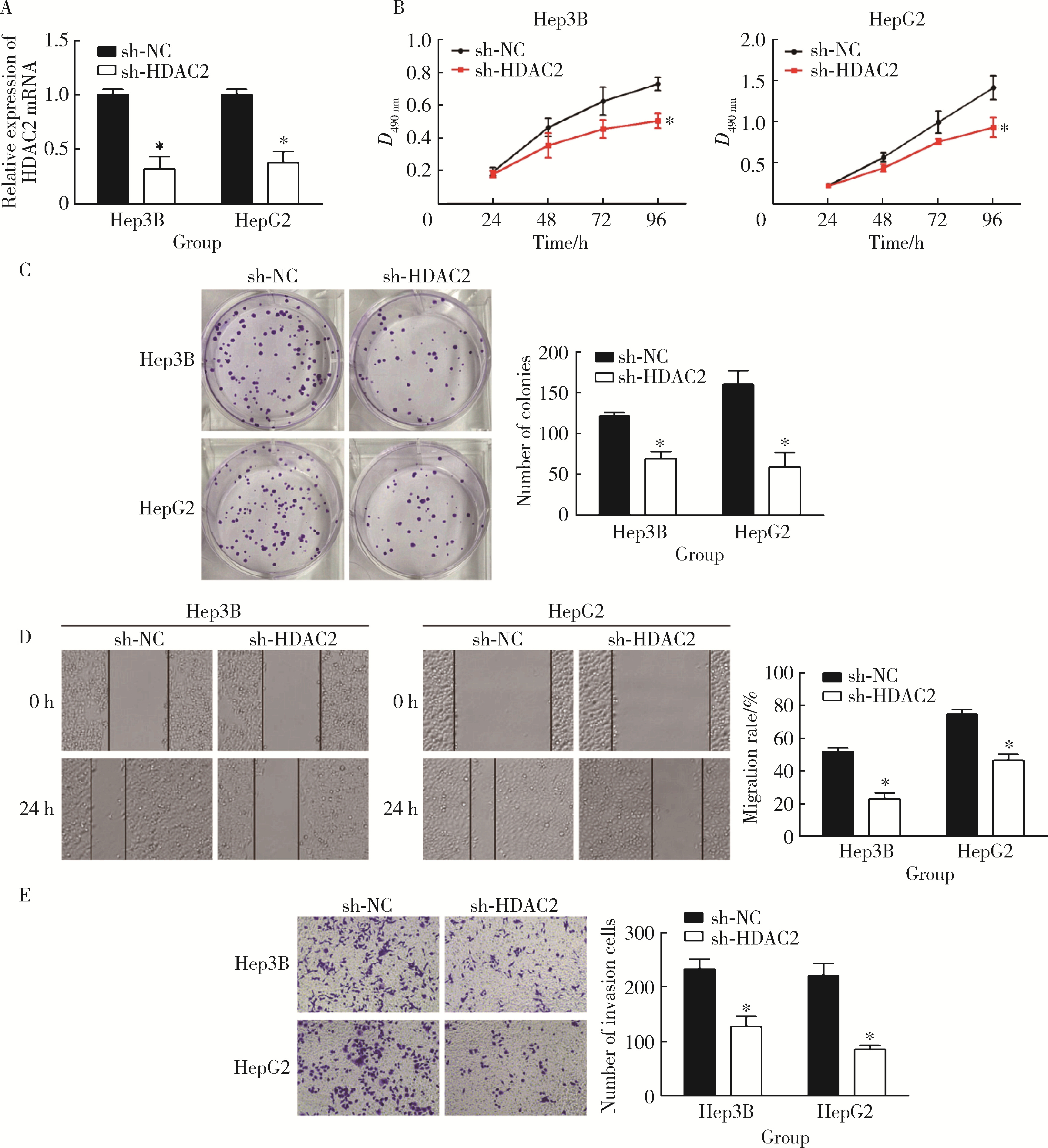
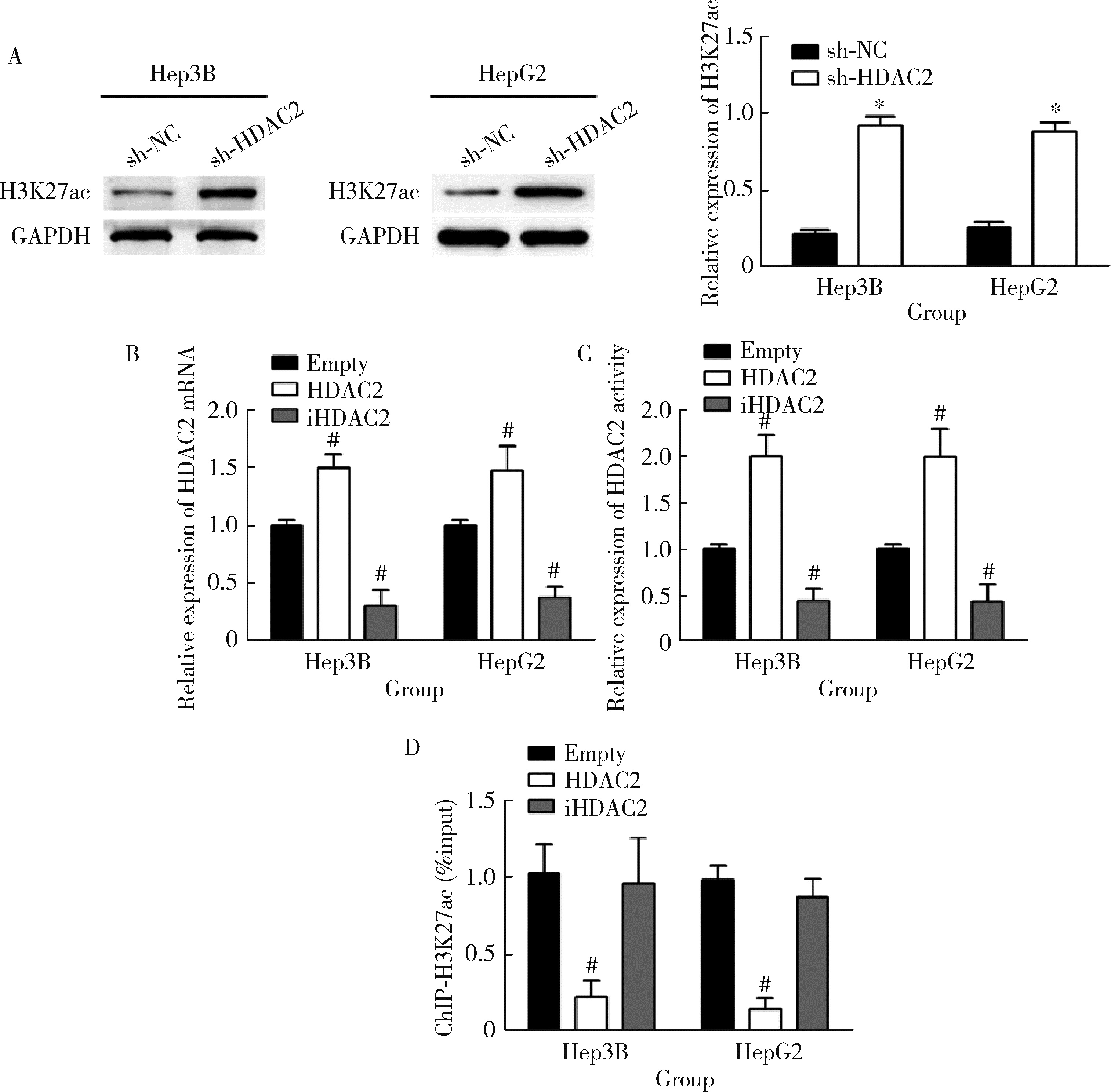
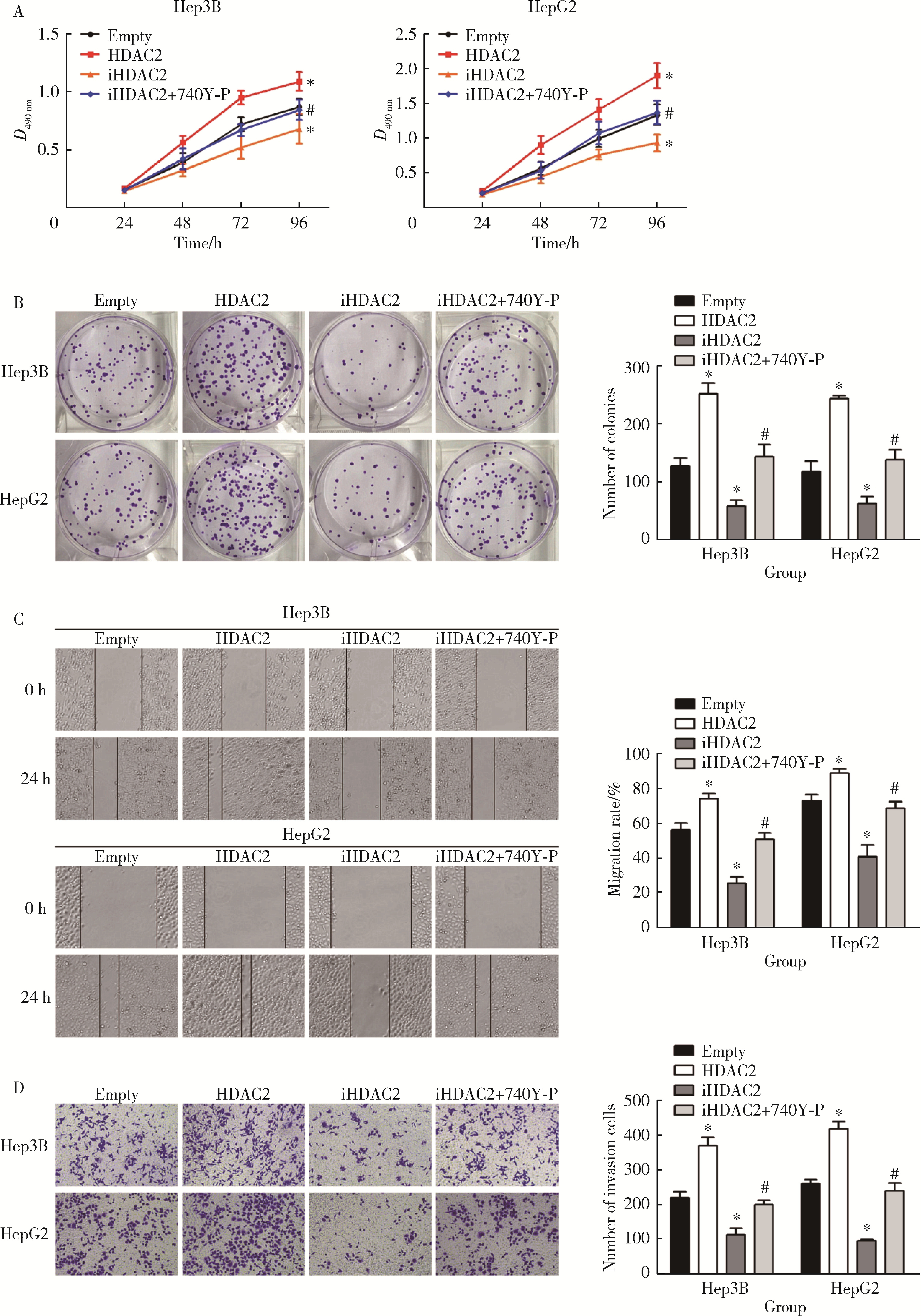
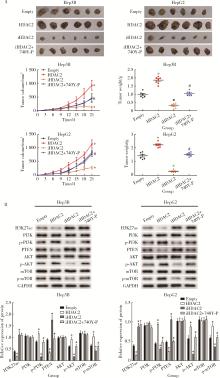
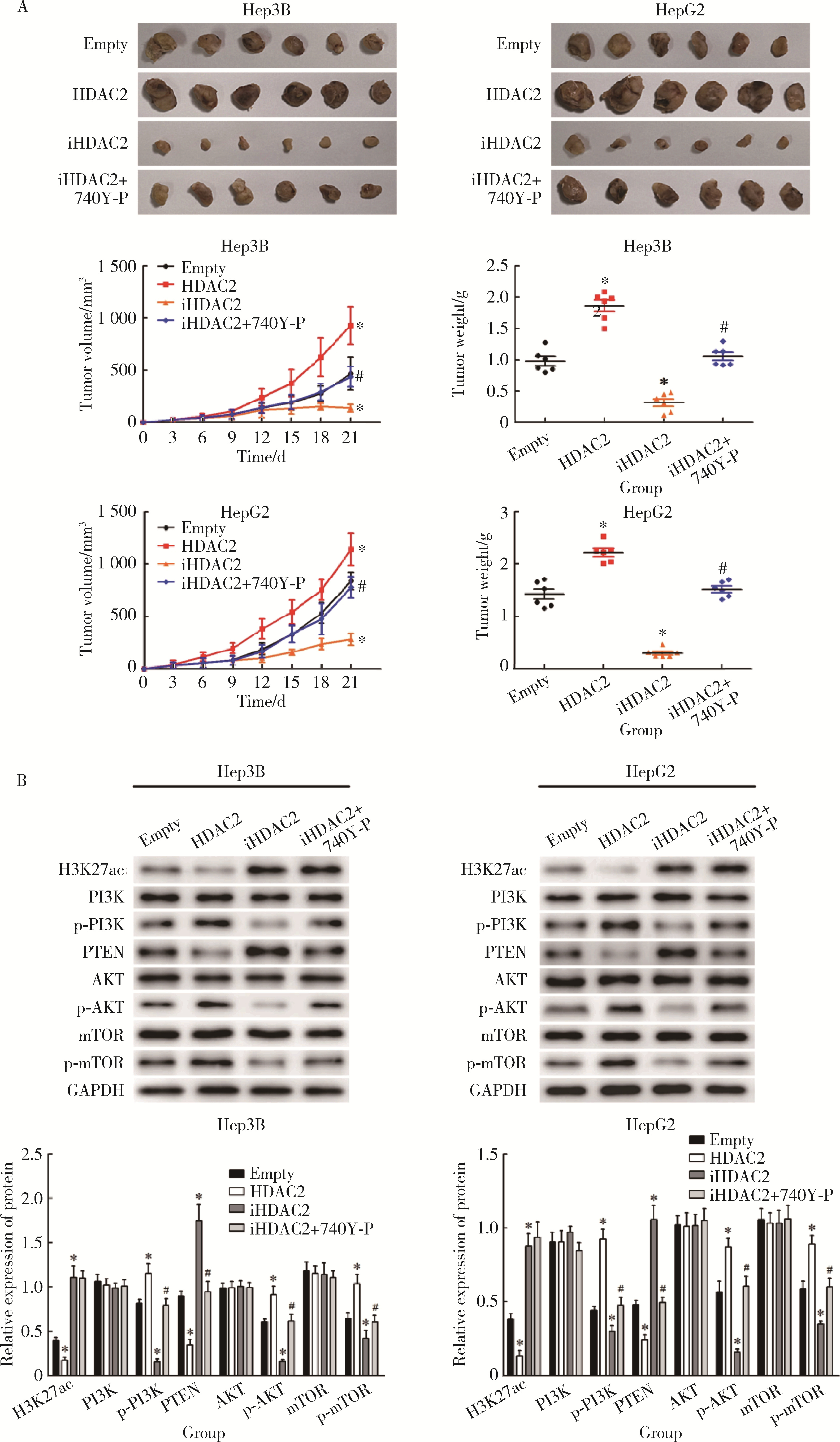
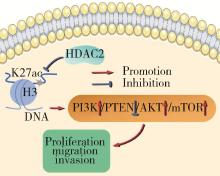
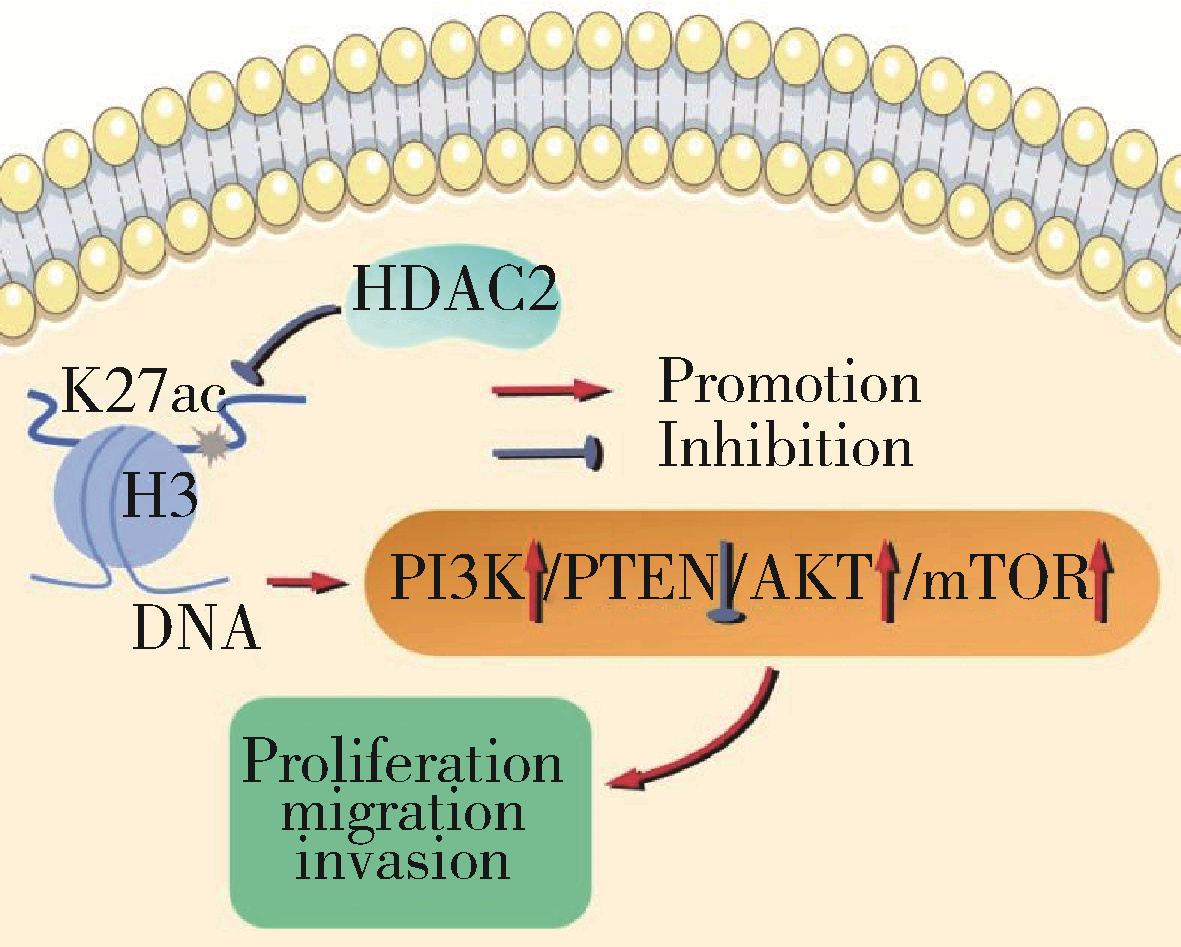
摘要: 目的: 探究组蛋白去乙酰化酶2(histone deacetylase 2, HDAC2)介导组蛋白H3第27位赖氨酸乙酰化(histone H3 lysine 27 acetylation, H3K27ac)修饰促进肝癌细胞增殖、迁移的作用机制。方法: 收集2021年1月至2023年1月经手术切除的40例肝癌、癌旁组织样本, 免疫组化和Western blotting检测肝癌、癌旁组织、细胞系HDAC2、H3K27ac表达, 分析HDAC2与H3K27ac表达水平的相关性, 以及HDAC2表达与肝癌患者临床病理特征的关系。Hep3B、HepG2细胞分为sh-NC组(转染sh-NC)、sh-HDAC2组(转染sh-HDAC2)、空质粒组(转染dCas9-空质粒)、HDAC2组(转染dCas9-HDAC2)、iHDAC2组(转染dCas9-失活HDAC2)和iHDAC2+740Y-P组(转染dCas9-失活HDAC2, 培养基加入30 μmol/mL 740Y-P)。通过MTS、克隆形成、划痕、Transwell实验测定各组Hep3B、HepG2细胞增殖、迁移、侵袭能力, 通过Western blotting、实时荧光定量PCR(real-time fluorescence quantitative PCR, qRT-PCR)、HDAC2活性检测、染色质免疫共沉淀-高通量测序(chromatin immunoprecipitation high-throughput sequencing, ChIP-seq)检测验证HDAC2介导H3K27ac修饰, 体内异种移植实验测定各组细胞成瘤能力, 检测磷脂酰肌醇-3激酶/第10号染色体磷酸酶和张力蛋白同源缺失基因/蛋白激酶B/哺乳动物雷帕霉素靶蛋白(phosphoinositide 3-kinases/phosphatase and tensin homolog deleted on chromosome ten/protein kinase B/mammalian target of rapamycin, PI3K/PTEN/AKT/mTOR)信号通路相关蛋白表达。结果: 肝癌组织、细胞系HDAC2呈高表达(P<0.05), H3K27ac呈低表达(P<0.05), 二者表达水平呈负相关(r=-0.477, P=0.002)。HDAC2表达水平与肿瘤大小、感染乙肝病毒、TNM分期、门静脉癌栓相关(P<0.05)。与Hep3B、HepG2细胞sh-NC组相比, sh-HDAC2组增殖、克隆形成、迁移、侵袭能力降低(P<0.05)。与空质粒组相比, HDAC2组HDAC2表达水平、活性、细胞增殖、克隆形成、迁移、侵袭能力, 体内成瘤体积、质量, p-PI3K、p-AKT、p-mTOR表达水平均升高(P<0.05), H3K27ac富集程度、H3K27ac、PTEN表达水平降低(P<0.05), iHDAC2组HDAC2表达水平、活性、增殖、克隆形成、迁移、侵袭能力, 体内成瘤体积、质量, p-PI3K、p-AKT、p-mTOR表达水平降低(P<0.05), H3K27ac、PTEN表达水平升高(P<0.05)。引入PI3K激活剂740Y-P验证PI3K/PTEN/AKT/mTOR信号通路参与HDAC2介导H3K27ac修饰的肝癌细胞恶性行为调控, 与iHDAC2组相比, iHDAC2+740Y-P组增殖、克隆形成、迁移、侵袭能力, 体内成瘤体积、质量, p-PI3K、p-AKT、p-mTOR表达水平升高(P<0.05), PTEN表达水平降低(P<0.05)。结论: HDAC2通过介导H3K27ac修饰启动PI3K/PTEN/AKT/mTOR信号通路, 促进肝癌发生发展。
中图分类号:
- R735.7
| 1 |
doi: 10.1038/s41568-021-00383-9 |
| 2 |
doi: 10.1016/j.cmet.2022.05.003 |
| 3 |
doi: 10.1080/17474124.2021.1991792 |
| 4 |
doi: 10.1002/hep.32740 |
| 5 |
doi: 10.3748/wjg.v28.i3.310 |
| 6 |
doi: 10.1186/s13046-021-01968-w |
| 7 |
doi: 10.1186/s12885-022-10247-6 |
| 8 |
doi: 10.3390/cancers13061265 |
| 9 |
doi: 10.1021/acs.jpcb.1c00694 |
| 10 |
doi: 10.1186/s13148-021-01126-1 |
| 11 |
doi: 10.1007/s12029-020-00370-7 |
| 12 |
doi: 10.3390/molecules27082568 |
| 13 |
|
| 14 |
|
| 15 |
doi: 10.1002/cmdc.202000643 |
| 16 |
姜健, 王维, 崔羽楠, 等. 基于2018版肝脏影像报告及数据系统评估CT及MRI对小于等于3 cm肝细胞性肝癌的诊断价值[J]. 磁共振成像, 2021, 12(9): 25-29, 44.
|
| 17 |
doi: 10.3390/genes12020208 |
| 18 |
doi: 10.1038/s12276-022-00812-1 |
| 19 |
doi: 10.1016/j.molcel.2021.12.004 |
| 20 |
|
| 21 |
doi: 10.1038/s41580-021-00441-y |
| 22 |
|
| 23 |
doi: 10.1002/tox.23516 |
| 24 |
doi: 10.1038/s41401-021-00765-7 |
| 25 |
doi: 10.3390/cancers14061538 |
| 26 |
doi: 10.1038/s41598-021-93815-3 |
| 27 |
doi: 10.1186/s12885-021-08114-x |
| 28 |
doi: 10.1515/biol-2021-0101 |
| 29 |
doi: 10.3892/ijmm.2021.4964 |
| 30 |
doi: 10.1002/tox.23802 |
| 31 |
doi: 10.1038/s41420-021-00750-3 |
| [1] | 张瑶,郭金鑫,战世佳,洪恩宇,杨慧,贾安娜,常艳,郭永丽,张璇. 富含半胱氨酸和甘氨酸蛋白2在神经母细胞瘤恶性进展中的功能和机制[J]. 北京大学学报(医学版), 2024, 56(3): 495-504. |
| [2] | 曹钟,岑红兵,赵建红,梅俊,秦灵芝,廖伟,敖启林. 胰腺神经内分泌肿瘤和实性假乳头状肿瘤中INSM1和SOX11的表达及意义[J]. 北京大学学报(医学版), 2023, 55(4): 575-581. |
| [3] | 王磊,韩天栋,江卫星,李钧,张道新,田野. 主动迁移技术与原位碎石技术在输尿管软镜治疗1~2 cm输尿管上段结石中的安全性和有效性比较[J]. 北京大学学报(医学版), 2023, 55(3): 553-557. |
| [4] | 刘媛,原婉琼,李婷,王平章,吕平,吴利新,阮国瑞,韩文玲,莫晓宁. 敲减CMTM3增加急性B淋巴细胞白血病细胞对伊马替尼敏感性[J]. 北京大学学报(医学版), 2022, 54(6): 1238-1243. |
| [5] | 杨朵,周心娜,王硕,王小利,袁艳华,杨化兵,耿会珍,彭兵,李子博,李彬,任军. 树突状细胞疫苗特异肿瘤多肽联合树突状细胞体外刺激淋巴细胞功能评估[J]. 北京大学学报(医学版), 2021, 53(6): 1094-1098. |
| [6] | 王子乔,刘燕鹰,张霞,刘田,任立敏,沈丹华,王屹,栗占国. 17例误诊为IgG4相关疾病患者的临床特点及误诊原因分析[J]. 北京大学学报(医学版), 2019, 51(6): 1025-1031. |
| [7] | 姚心韵,高晓敏,邹晓英,岳林. 胞内转运对根尖牙乳头干细胞表面CXC趋化因子受体4表达的影响[J]. 北京大学学报(医学版), 2019, 51(5): 893-899. |
| [8] | 谢静,赵玉鸣,饶南荃,汪晓彤,方滕姣子,李晓霞,翟越,李静芝,葛立宏,王媛媛. 3种口腔颌面部来源的间充质干细胞成血管内皮分化潜能的比较研究[J]. 北京大学学报(医学版), 2019, 51(5): 900-906. |
| [9] | 张帆,燕太强,郭卫. Rasfonin抑制骨肉瘤细胞143B的增殖和迁移[J]. 北京大学学报(医学版), 2019, 51(2): 234-238. |
| [10] | 季兰岚,郝燕捷,张卓莉. 原发性骨髓纤维化引起的继发性痛风1例[J]. 北京大学学报(医学版), 2018, 50(6): 1117-1119. |
| [11] | 王子成,程立,吕同德,苏黎,林健,周利群. 炎症因子预处理的脂肪干细胞可明显抑制外周血单个核细胞增殖[J]. 北京大学学报(医学版), 2018, 50(4): 590-594. |
| [12] | 唐旭,赵卫红,宋琴琴,殷华奇,杜依青,盛正祚,王强,张晓威,李清,刘士军,徐涛. SOX10对前列腺癌细胞增殖及侵袭的影响[J]. 北京大学学报(医学版), 2018, 50(4): 602-606. |
| [13] | 汪晓彤,饶南荃,方腾姣子,赵玉鸣,葛立宏. 乳牙牙髓干细胞CD146阳性/阴性细胞亚群生物学特性的比较[J]. 北京大学学报(医学版), 2018, 50(2): 284-292. |
| [14] | 陈玮, 胡凡磊, 刘洪江, 徐丽玲, 李英妮, 栗占国. 类风湿关节炎患者髓系来源的抑制细胞促进自身B细胞增殖[J]. 北京大学学报(医学版), 2017, 49(5): 819-823. |
| [15] | 蔡燚,郭浩,李汉忠,王文达,张玉石. 结节性硬化症细胞株TSC2-/- MEFs和正常细胞株TSC2+/+ MEFs微小RNA表达谱的差异分析[J]. 北京大学学报(医学版), 2017, 49(4): 580-584. |
|
||