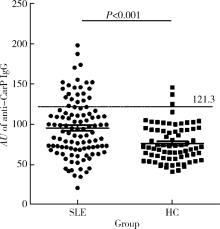
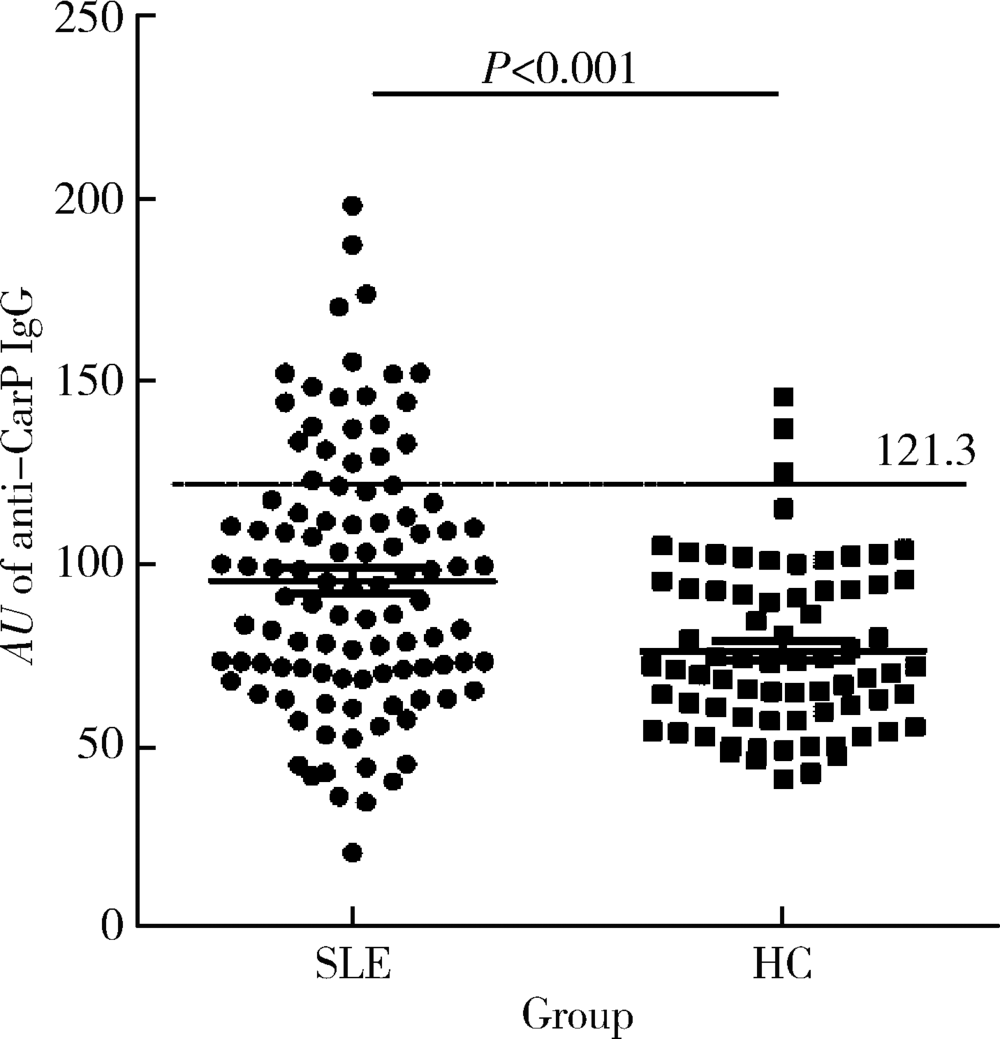
Journal of Peking University(Health Sciences) ›› 2019, Vol. 51 ›› Issue (6): 1019-1024. doi: 10.19723/j.issn.1671-167X.2019.06.007
Previous Articles Next Articles
Significance of anti-carbamylated fibrinogen antibodies in systemic lupus erythematosus
Ying-ni LI,Xiao-hong XIANG,Jing ZHAO,Yun LI,Feng SUN,Hong-yan WANG,Ru-lin JIA,Fan-lei HU( )
)
- Department of Rheumatology & Immunology, Peking University People’s Hospital, Beijing 100044, China
CLC Number:
- R593.24
| [1] | Lee SJ, Silverman E, Bargman JM . The role of antimalarial agents in the treatment of SLE and lupus nephritis[J]. Nat Rev Nephrol, 2011,7(12):718-729. |
| [2] | Yaniv G, Twig G, Shor DB , et al. A volcanic explosion of autoantibodies in systemic lupus erythematosus: a diversity of 180 different antibodies found in SLE patients[J]. Autoimmun Rev, 2015,14(1):75-79. |
| [3] | Sherer Y, Gorstein A, Fritzler MJ , et al. Autoantibody explosion in systemic lupus erythematosus: more than 100 different anti-bodies found in SLE patients[J]. Semin Arthritis Rheum, 2004,34(2):501-537. |
| [4] | Betancur JF, Gómez-Puerta JA . Antinuclear antibodies mitotic patterns and their clinical associations [J/OL]. Ann Rheum Dis ( 2019-04-29)[2019-08-01]. |
| [5] | Bizzaro N, Villalta D, Giavarina D , et al. Are anti-nucleosome antibodies a better diagnostic marker than anti-dsDNA antibodies for systemic lupus erythematosus? A systematic review and a study of metanalysis[J]. Autoimmun Rev, 2012,12(2):97-106. |
| [6] | Schreier SM, Steinkellner H, Jirovetz L , et al. S-carbamoylation impairs the oxidant scavenging activity of cysteine: its possible impact on increased LDL modification in uraemia[J]. Biochimie, 2011,93(4):772-777. |
| [7] | Gross ML, Piecha G, Bierhaus A , et al. Glycated and carbamylated albumin are more “nephrotoxic” than unmodified albumin in the amphibian kidney[J]. Am J Physiol Renal Physiol, 2011,301(3):476-485. |
| [8] | Trepanier DJ, Thibert RJ . Carbamylation of erythrocyte membrane aminophospholipids: an in vitro and in vivo study[J]. Clin Biochem, 1996,29(4):333-345. |
| [9] | Shi J, Knevel R, Suwannalai P , et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage[J]. Proc Natl Acad Sci USA, 2011,108(42):17372-17377. |
| [10] | Bergum B, Koro C, Delaleu N , et al. Antibodies against carbamylated proteins are present in primary Sjögren’s syndrome and are associated with disease severity[J]. Ann Rheum Dis, 2016,75(8):1494-1500. |
| [11] | Scinocca M, Bell DA, Racapé M , et al. Antihomocitrullinated fibrinogen antibodies are specific to rheumatoid arthritis and frequently bind citrullinated proteins/peptides[J]. J Rheumatol, 2014,41(2):270-279. |
| [12] | López-Hoyos M, Álvarez-Rodríguez L, Mahler M , et al. Anti-carbamylated protein antibodies in patients with ageing associated inflammatory chronic disorders[J]. Rheumatology (Oxford), 2016,55(4):764-766. |
| [13] | Ziegelasch M, van Delft MA, Wallin P , et al. Antibodies against carbamylated proteins and cyclic citrullinated peptides in systemic lupus erythematosus: results from two welldefined European cohorts[J]. Arthritis Res Ther, 2016,18(1):289. |
| [14] | Nakabo S, Yoshifuji H, Hashimoto M , et al. Anti-carbamylated protein antibodies are detectable in various connective tissue dis-eases[J]. J Rheumatol, 2017,44(9):1384-1388. |
| [15] | Hochberg MC . Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus[J]. Arthritis Rheum, 1997,40(9):1725. |
| [16] | Turunen S, Koivula MK, Risteli L , et al. Anticitrulline antibodies can be caused by homocitrulline-containing proteins in rabbits[J]. Arthritis Rheum, 2010,62(11):3345-3352. |
| [17] | Shi J, van de Stadt LA, Levarht EWT , et al. Anti-carbamylated protein (anti-CarP) antibodies precede the onset of rheumatoid arthritis[J]. Ann Rheum Dis, 2014,73(4):780-783. |
| [18] | Ceccarelli F, Perricone C, Colasanti T , et al. Anti-carbamylated protein antibodies as a new biomarker of erosive joint damage in systemic lupus erythematosus[J]. Arthritis Res Ther, 2018,20(1):126 |
| [19] | Ceccarelli F, Perricone C, Cipriano E , et al. Joint involvement in systemic lupus erythematosus: from pathogenesis to clinical assessment[J]. Semin Arthritis Rheum, 2017,47(1):53-64. |
| [20] | Mastrangelo A, Colasanti T, Barbati C , et al. The role of post-translational protein modifications in rheumatological diseases: Focus on rheumatoid arthritis [J/OL]. J Immunol Res, 2015, 2015: 712490(2015-05-18)[2019-08-01]. |
| [1] | Zhihui WU, Mingzhi HU, Qiaoying ZHAO, Fengfeng LV, Jingying ZHANG, Wei ZHANG, Yongfu WANG, Xiaolin SUN, Hui WANG. Immunomodulatory mechanism of umbilical cord mesenchymal stem cells modified by miR-125b-5p in systemic lupus erythematosus [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 860-867. |
| [2] | Limin REN,Chuchu ZHAO,Yi ZHAO,Huiqiong ZHOU,Liyun ZHANG,Youlian WANG,Lingxun SHEN,Wenqiang FAN,Yang LI,Xiaomei LI,Jibo WANG,Yongjing CHENG,Jiajing PENG,Xiaozhen ZHAO,Miao SHAO,Ru Li. Low disease activity and remission status of systemic lupus erythematosus in a real-world study [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 273-278. |
| [3] | Xiang-ge ZHAO,Jia-qing LIU,Hui-na HUANG,Zhi-min LU,Zi-ran BAI,Xia LI,Jing-jing QI. Interferon-α mediating the functional damage of CD56dimCD57+natural killer cells in peripheral blood of systemic lupus erythematosuss [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 975-981. |
| [4] | Hai-hong YAO,Fan YANG,Su-mei TANG,Xia ZHANG,Jing HE,Yuan JIA. Clinical characteristics and diagnostic indicators of macrophage activation syndrome in patients with systemic lupus erythematosus and adult-onset Still's disease [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 966-974. |
| [5] | Zhi-jun LUO,Jia-jia WU,You SONG,Chun-li MEI,Rong DU. Systemic lupus erythematosus associated macrophage activation syndrome with neuropsychiatric symptoms: A report of 2 cases [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1111-1117. |
| [6] | Miao SHAO,Hui-fang GUO,Ling-yan LEI,Qing ZHAO,Yan-jie DING,Jin LIN,Rui WU,Feng YU,Yu-cui LI,Hua-li MIAO,Li-yun ZHANG,Yan DU,Rui-ying JIAO,Li-xia PANG,Li LONG,Zhan-guo LI,Ru LI. A multicenter study on the tolerance of intravenous low-dose cyclophosphamide in systemic lupus erythematosus [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1112-1116. |
| [7] | Min LI,Lin-qing HOU,Yue-bo JIN,Jing HE. Clinical and immunological characteristics of systemic lupus erythematosus with retinopathy [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1106-1111. |
| [8] | Lin-qi ZHANG,Jing ZHAO,Hong-yan WANG,Zong-yi WANG,Ying-ni LI,Ji-yang TANG,Si-ying LI,Jin-feng QU,Ming-wei ZHAO. Relationship between anti-ENO1 antibody and systemic lupus erythematosus patients with retinopathy [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1099-1105. |
| [9] | Jian-mei ZOU,Li-jun WU,Cai-nan LUO,Ya-mei SHI,Xue WU. Relationship of serum 25- hydroxy vitamin D and systemic lupus erythematosus [J]. Journal of Peking University (Health Sciences), 2021, 53(5): 938-941. |
| [10] | XIA Fang-fang,LU Fu-ai,LV Hui-min,YANG Guo-an,LIU Yuan. Clinical characteristics and related factors of systemic lupus erythematosus with interstitial pneumonia [J]. Journal of Peking University (Health Sciences), 2021, 53(2): 266-272. |
| [11] | Yan GENG,Bo-rui LI,Zhuo-li ZHANG. Musculoskeletal ultrasound findings of symptomatic joints in patients with systemic lupus erythematosus [J]. Journal of Peking University(Health Sciences), 2020, 52(1): 163-168. |
| [12] | Yu-hua WANG,Guo-hua ZHANG,Ling-ling ZHANG,Jun-li LUO,Lan GAO. Adrenal hemorrhage in a patient with systemic lupus erythematosus [J]. Journal of Peking University(Health Sciences), 2019, 51(6): 1178-1181. |
| [13] | Fan YANG,Yun-shan ZHOU,Yuan JIA. Systemic lupus erythematosus with acquired hemophilia A: a case report [J]. Journal of Peking University(Health Sciences), 2018, 50(6): 1108-1111. |
| [14] | Xiao-hui ZHANG,Xue-rong DENG,Fan Li,Ying ZHU,Zhuo-li ZHANG. Posterior reversible encephalopathy syndrome in systemic lupus erythematosus: a case report [J]. Journal of Peking University(Health Sciences), 2018, 50(6): 1102-1107. |
| [15] | Shuang LIU,Yu-long GUO,Jing-yi YANG,Wei WANG,Jian XU. Efficacy of mesenchymal stem cells on systemic lupus erythematosus:a meta-analysis [J]. Journal of Peking University(Health Sciences), 2018, 50(6): 1014-1021. |
|
||