Journal of Peking University(Health Sciences) ›› 2019, Vol. 51 ›› Issue (6): 989-995. doi: 10.19723/j.issn.1671-167X.2019.06.002
Previous Articles Next Articles
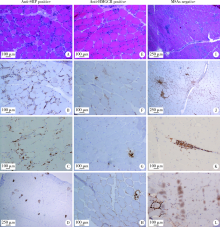
Clinical and pathological characteristics of immune mediated necrotizing myopathy
Hong-xia YANG1,2,Xiao-lan TIAN2,Wei JIANG2,Wen-li LI2,Qing-yan LIU2,Qing-lin PENG2,Guo-chun WANG2,Xin LU1,△( )
)
- 1. Peking University China-Japan Friendship School of Clinical Medicine, Beijing 100029, China
2. Department of Rheumatology, China-Japan Friendship Hospital, Beijing 100029, China
CLC Number:
- R593.26
| [1] | Hoogendijk JE, Amato AA, Lecky BR , et al. 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies,with the exception of inclusion body myositis[J]. Neuromuscul Disord, 2004,14(5):337-345. |
| [2] | Allenbach Y, Mammen AL, Benveniste O , et al. 224th ENMC International Workshop: clinico-sero-pathological classification of immune-mediated necrotizing myopathies[J]. Neuromuscul Disord, 2018,28(1):87-99. |
| [3] | Pinal-Fernandez I, Mammen AL . Spectrum of immune-mediated necrotizing myopathies and their treatments[J]. Curr Opin Rheumatol, 2016,28(6):619-624. |
| [4] | Lim J, Rietveld A, De Bleecker JL , et al. Seronegative patients form a distinctive subgroup of immune-mediated necrotizing myopathy[J]. Neurol Neuroimmunol Neuroinflamm, 2018,6(1):e513. |
| [5] | Bohan A, Peter JB . Polymyositis and dermatomyositis (first of two parts)[J]. N Engl J Med, 1975,292(7):344-347. |
| [6] | Bohan A, Peter JB . Polymyositis and dermatomyositis (second of two parts)[J]. N Engl J Med, 1975,292(8):403-407. |
| [7] | Suzuki S, Nishikawa A, Kuwana M , et al. Inflammatory myopathy with anti-signal recognition particle antibodies: case series of 100 patients[J]. Orphanet J Rare Dis, 2015,5(13):10-61. |
| [8] | Suzuki S, Hayashi YK, Kuwana M , et al. Myopathy associated with anti- bodies to signal recognition particle: disease progression and neurological outcome[J]. Arch Neurol, 2012,69(6):728-732. |
| [9] | Raghu G, Collard HR, Egan JJ , et al. An official ATSERSJRSALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management[J]. Am J Respir Crit Care Med, 2011,183(6):788-824. |
| [10] | Travis WD, Costabel U, Hansell DM , et al. An official American Thoracic Society European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias[J]. Am J Respir Crit Care Med, 2013,188(6):733-748. |
| [11] | Dalakas MC . Polymyositis, dermatomyositis and inclusion-body myositis[J]. N Engl J Med, 1991,325(21):1487-1498. |
| [12] | Watanabe Y, Uruha A, Suzuki S , et al. Clinical features and prognosis in anti-SRP and anti-HMGCR necrotising myopathy[J]. Neurol Neurosurg Psychiatry, 2016,87(10):1038-1044. |
| [13] | Takada T, Hirakata M, Suwa A , et al. Clinical and histopatholo-gical features of myopathies in Japanese patients with anti-SRP autoantibodies[J]. Mod Rheumatol, 2009,19(2):156-164. |
| [14] | Ge Y, Lu X, Peng Q , et al. Clinical characteristics of anti-3-hydroxy-3-methylglutaryl coenzyme A reductase antibodies in Chinese patients with idiopathic inflammatory myopathies[J]. PLoS One, 2015,10(10):e0141616. |
| [15] | Limaye V, Bundell C, Hollingsworth P , et al. Clinical and gene-tic associations of autoantibodies to 3-hydroxy-3-methyl-glutaryl-coenzyme a reductase in patients with immune-mediated myositis and necrotizing myopathy[J]. Muscle Nerve, 2015,52(2):196-203. |
| [16] | Christopher-Stine L, Casciola-Rosen LA, Hong G , et al. A novel autoantibody recognizing 200-kD and 100-kD proteins is associated with an immune-mediated necrotizing myopathy[J]. Arthritis Rheum, 2010,62(9):2757-2766. |
| [17] | Mammens AL . Which nonautoimmune myopathies are most frequently misdiagnosed as myositis?[J]. Curr Opin Rheumatol, 2017,29(6):618-622. |
| [18] | Wang Q, Li Y, Ji SQ , et al. Immunopathological characterization of muscle biopsy samples from immune-mediated necrotizing myo-pathy patients[J]. Med Sci Monit, 2018,12(24):2189-2196. |
| [19] | Wang L, Liu LL, Hao HJ , et al. Myopathy with anti-signal recognition particle antibodies: clinical and histopathological features in Chinese patients[J]. Neuromuscular Disorders, 2014,24(4):335-341. |
| [1] | Dongwu LIU, Jie CHEN, Mingli GAO, Jing YU. Rheumatoid arthritis with Castleman-like histopathology in lymph nodes: A case report [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 928-931. |
| [2] | Yun-fei SHI,Hao-jie WANG,Wei-ping LIU,Lan MI,Meng-ping LONG,Yan-fei LIU,Yu-mei LAI,Li-xin ZHOU,Xin-ting DIAO,Xiang-hong LI. Analysis of clinicopathological and molecular abnormalities of angioimmunoblastic T-cell lymphoma [J]. Journal of Peking University (Health Sciences), 2023, 55(3): 521-529. |
| [3] | Qi SHEN,Yi-xiao LIU,Qun HE. Mucinous tubular and spindle cell carcinoma of kidney: Clinicopathology and prognosis [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 276-282. |
| [4] | Wei-hua HOU,Shu-jie SONG,Zhong-yue SHI,Mu-lan JIN. Clinicopathological features of Helicobacter pylori-negative early gastric cancer [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 292-298. |
| [5] | Xue-mei HA,Yong-zheng YAO,Li-hua SUN,Chun-yang XIN,Yan XIONG. Solid placental transmogrification of the lung: A case report and literature review [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 357-361. |
| [6] | Bo-han NING,Qing-xia ZHANG,Hui YANG,Ying DONG. Endometrioid adenocarcinoma with proliferated stromal cells, hyalinization and cord-like formations: A case report [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 366-369. |
| [7] | Qian SU,Xin PENG,Chuan-xiang ZHOU,Guang-yan YU. Clinicopathological characteristics and prognosis of non-Hodgkin lymphoma in oral and maxillofacial regions: An analysis of 369 cases [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 13-21. |
| [8] | Xiao-yan XING,Jun-xiao ZHANG,Feng-yun-zhi ZHU,Yi-fan WANG,Xin-yao ZHOU,Yu-hui LI. Clinical analysis of 5 cases of dermatomyositis complicated with macrophage activation syndrome [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1214-1218. |
| [9] | Er-shu BO,Peng HONG,Yu ZHANG,Shao-hui DENG,Li-yuan GE,Min LU,Nan LI,Lu-lin MA,Shu-dong ZHANG. Clinicopathological features and prognostic analysis of papillary renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2022, 54(4): 615-620. |
| [10] | Yi-cen YING,Qi TANG,Kai-wei YANG,Yue MI,Yu FAN,Wei YU,Yi SONG,Zhi-song HE,Li-qun ZHOU,Xue-song LI. Clinical features of immune checkpoint inhibitor-related myositis in patients with urological cancer [J]. Journal of Peking University (Health Sciences), 2022, 54(4): 644-651. |
| [11] | LI Bing-yu,TANG Zu-nan,HU Lei-hao,ZHANG Wen-bo,YU Yao,YU Guang-yan,PENG Xin. Clinicopathologic analysis of micro and mini parotid gland tumors [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 335-339. |
| [12] | XUE Jiang,ZHANG Jian-yun,SHI Rui-rui,XIE Xiao-yan,BAI Jia-ying,LI Tie-jun. Clinicopathological analysis of 105 patients with fibrous dysplasia of cranio-maxillofacial region [J]. Journal of Peking University (Health Sciences), 2022, 54(1): 54-61. |
| [13] | ZHANG Pu-li,YANG Hong-xia,ZHANG Li-ning,GE Yong-peng,PENG Qing-lin,WANG Guo-chun,LU Xin. Value of serum YKL-40 in the diagnosis of anti-MDA5-positive patients with dermatomyositis complicated with severe pulmonary injury [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1055-1060. |
| [14] | LUO Lan,XING Xiao-yan,XIAO Yun-shu,CHEN Ke-yan,ZHU Feng-yun-zhi,ZHANG Xue-wu,LI Yu-hui. Clinical and immunological characteristics of patients with anti-synthetase syndrome complicated with cardiac involvement [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1078-1082. |
| [15] | XIAO Yun-shu,ZHU Feng-yun-zhi,LUO Lan,XING Xiao-yan,LI Yu-hui,ZHANG Xue-wu,SHEN Dan-hua. Clinical and immunological characteristics of 88 cases of overlap myositis [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1088-1093. |
|
||