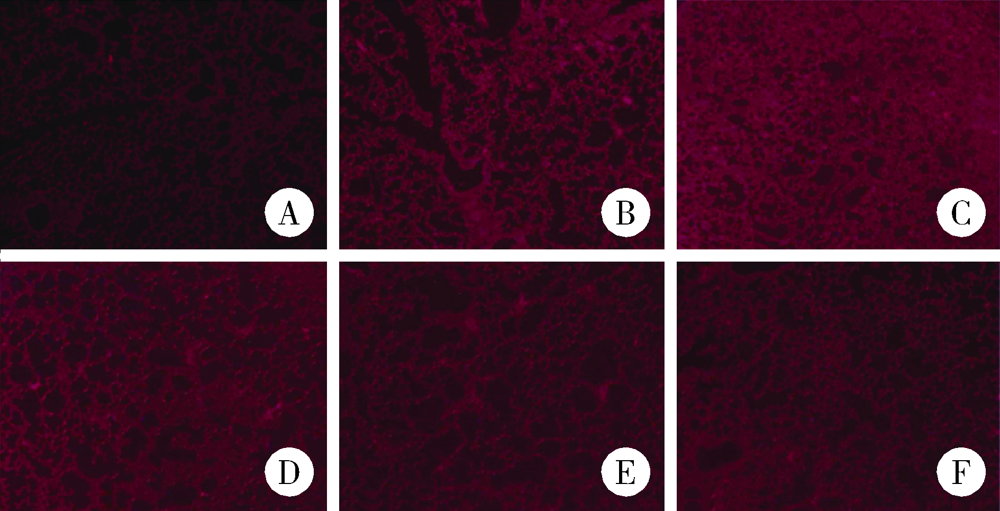
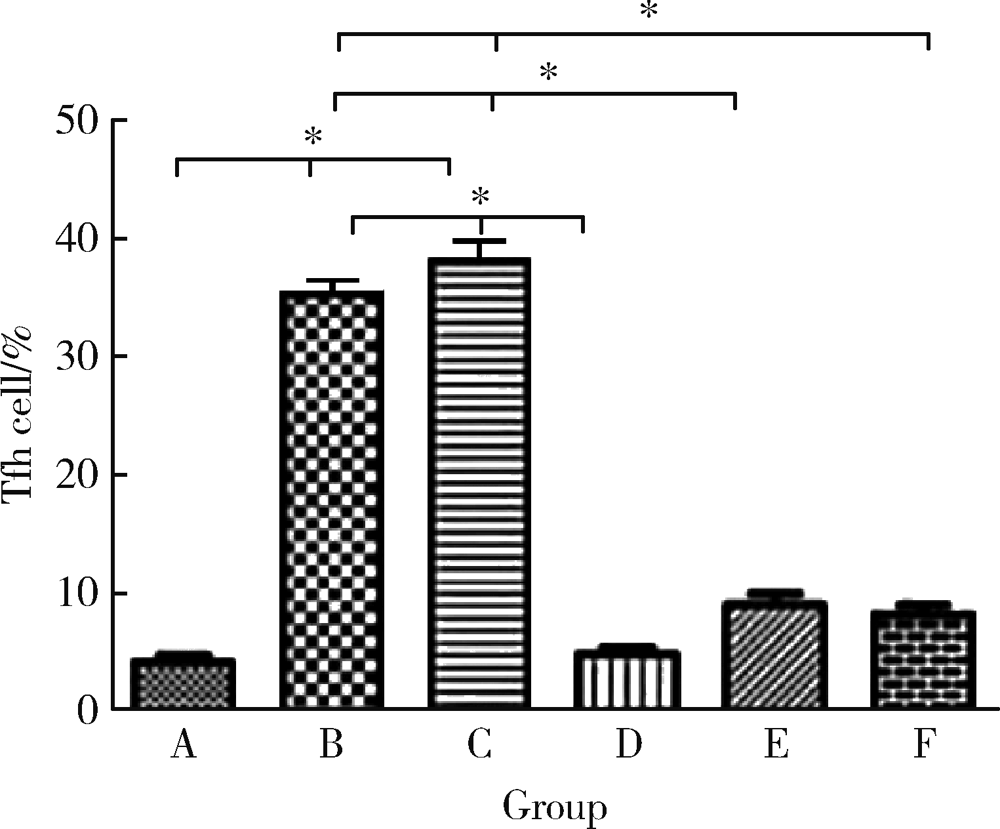
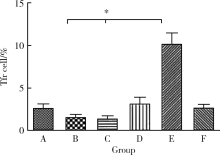
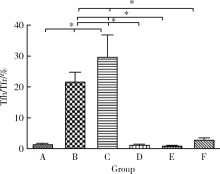
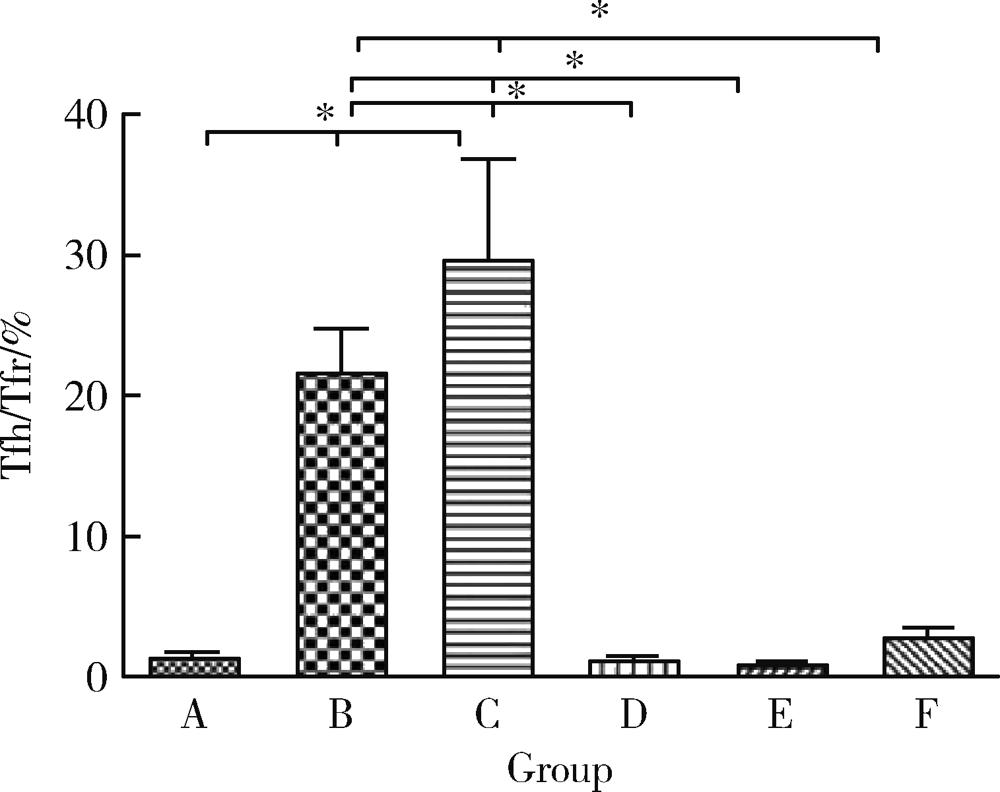
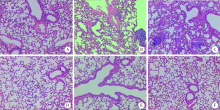
Journal of Peking University (Health Sciences) ›› 2021, Vol. 53 ›› Issue (2): 235-239. doi: 10.19723/j.issn.1671-167X.2021.02.001
Therapeutic effect of gene silencing peptidyl arginine deaminase 4 on pulmonary interstitial lesions induced by collagen-induced arthritis mice
ZHAO Kai,CHANG Zhi-fang,WANG Zhi-hua,PANG Chun-yan( ),WANG Yong-fu(
),WANG Yong-fu( )
)
- Department of Rheumatism and Immunology, the First Affiliated Hospital of Baotou Medical College, Inner Monolia University of Secience and Technology, Inner Mongolia Autoimmunology Key Laboratory, Baotou 014010, Inner Mongolia, China
CLC Number:
- R593.22
| [1] |
Haridas V, Shetty P, Sarathkumar E, et al. Reciprocal regulation of pro-inflammatory Annexin A2 and anti-inflammatory Annexin A1 in the pathogenesis of rheumatoid arthritis[J]. Mol Biol Rep, 2019,46(1):83-95.
doi: 10.1007/s11033-018-4448-5 pmid: 30426384 |
| [2] |
Yuzhalin AE, Gordon-Weeks AN, Tognoli ML, et al. Colorectal cancer liver metastatic growth depends on PAD4-driver citrullination of the extracellular matrix[J]. Nat Commun, 2018,9(1):4783.
pmid: 30429478 |
| [3] | 郭靖, 钱龙, 李向培, 等. 类风湿关节炎患者外周血单个核细胞肽酰基精氨酸脱亚氨酶4表达及组蛋白甲基化水平[J]. 中华内科杂志, 2013,52(11):928-931. |
| [4] |
Thanapati S, Ganu M, Giri P, et al. Impaired NK cell functiona-lity and increased TNF-α production as biomarkers of chronic chikungunya arthritis and rheumatoid arthritis[J]. Hum Immunol, 2017,78(4):370-374.
pmid: 28213049 |
| [5] | 王建. Th细胞在类风湿关节炎发病作用的研究进展[J]. 临床与病理杂志, 2015,35(2):263-266. |
| [6] |
Xu B, Li J, Wu C, et al. CXCL10 and TRAIL are upregulated by TXNDC5 in rheumatoid arthritis fibroblast-like synoviocytes[J]. J Rheumatol, 2018,45(3):335-340.
doi: 10.3899/jrheum.170170 pmid: 29247155 |
| [7] | 刘倩, 杨宇, 陈立, 等. 血小板源性生长因子及其受体在类风湿性关节炎大鼠肺组织的表达及与风湿肺关系的探讨[J]. 中国医科大学学报, 2002,31(1):11-14. |
| [8] |
Darrah E, Giles JT, Davis RL, et al. Autoantibodies to peptidylarginine deiminase 2 are associated with less severe disease in rheumatoid arthritis[J]. Front Immunol, 2018,9:2696.
doi: 10.3389/fimmu.2018.02696 pmid: 30515171 |
| [9] |
Apa I, Saliba D, Ponzoni M, et al. TFH-derived dopamine accelelates productive synapses in germinal centres[J]. Nature, 2017,547(7663):318-323.
doi: 10.1038/nature23013 pmid: 28700579 |
| [10] | 邹晓月, 熊御云, 张龙锋, 等. 类风湿关节炎患者外周血滤泡辅助性T淋巴细胞百分率的变化及意义[J]. 重庆医学, 2017,46(35):4920-4922. |
| [11] | Wang X, Yang C, Xu F, et al. Imbalance of circulating Tfr/Tfh ratio in patients with rheumatoid arthritis[J]. Clin Exp Med, 2019,19(1):55-64. |
| [12] | Hou S, Clement RL, Diallo A, et al. FoxP3 and Ezh2 regulate Tfr cell suppressive function and transcriptional program[J]. J Exp Med, 2019,216(3):605-620. |
| [1] | Dongwu LIU, Jie CHEN, Mingli GAO, Jing YU. Rheumatoid arthritis with Castleman-like histopathology in lymph nodes: A case report [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 928-931. |
| [2] | Huina HUANG,Jing ZHAO,Xiangge ZHAO,Ziran BAI,Xia LI,Guan WANG. Regulatory effect of lactate on peripheral blood CD4+ T cell subsets in patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 519-525. |
| [3] | Xiaofei TANG,Yonghong LI,Qiuling DING,Zhuo SUN,Yang ZHANG,Yumei WANG,Meiyi TIAN,Jian LIU. Incidence and risk factors of deep vein thrombosis in patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 279-283. |
| [4] | Xue ZOU,Xiao-juan BAI,Li-qing ZHANG. Effectiveness of tofacitinib combined with iguratimod in the treatment of difficult-to-treat moderate-to-severe rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1013-1021. |
| [5] | Qi WU,Yue-ming CAI,Juan HE,Wen-di HUANG,Qing-wen WANG. Correlation between dyslipidemia and rheumatoid arthritis associated interstitial lung disease [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 982-992. |
| [6] | Jing-feng ZHANG,Yin-ji JIN,Hui WEI,Zhong-qiang YAO,Jin-xia ZHAO. Correlation analysis between body mass index and clinical characteristics of rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 993-999. |
| [7] | Yin-ji JIN,Lin SUN,Jin-xia ZHAO,Xiang-yuan LIU. Significance of IgA isotype of anti-v-raf murine sarcoma viral oncogene homologue B1 antibody in rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 631-635. |
| [8] | Wen-xin CAI,Shi-cheng LI,Yi-ming LIU,Ru-yu LIANG,Jing LI,Jian-ping GUO,Fan-lei HU,Xiao-lin SUN,Chun LI,Xu LIU,Hua YE,Li-zong DENG,Ru LI,Zhan-guo LI. A cross-sectional study on the clinical phenotypes of rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1068-1073. |
| [9] | Fang CHENG,Shao-ying YANG,Xing-xing FANG,Xuan WANG,Fu-tao ZHAO. Role of the CCL28-CCR10 pathway in monocyte migration in rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1074-1078. |
| [10] | Rui LIU,Jin-xia ZHAO,Liang YAN. Clinical characteristics of patients with rheumatoid arthritis complicated with venous thrombosis of lower extremities [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1079-1085. |
| [11] | Jing-feng ZHANG,Yin-ji JIN,Hui WEI,Zhong-qiang YAO,Jin-xia ZHAO. Cross-sectional study on quality of life and disease activity of rheumatoid arthritis patients [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1086-1093. |
| [12] | GAO Chao,CHEN Li-hong,WANG Li,YAO Hong,HUANG Xiao-wei,JIA Yu-bo,LIU Tian. Validation of the Pollard’s classification criteria (2010) for rheumatoid arthritis patients with fibromyalgia [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 278-282. |
| [13] | ZHONG Hua,XU Li-ling,BAI Ming-xin,SU Yin. Effect of chemokines CXCL9 and CXCL10 on bone erosion in patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1026-1031. |
| [14] | LOU Xue,LIAO Li,LI Xing-jun,WANG Nan,LIU Shuang,CUI Ruo-mei,XU Jian. Methylation status and expression of TWEAK gene promoter region in peripheral blood of patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1020-1025. |
| [15] | LUO Liang,HUO Wen-gang,ZHANG Qin,LI Chun. Clinical characteristics and risk factors of rheumatoid arthritis with ulcerative keratitis [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1032-1036. |
|
||