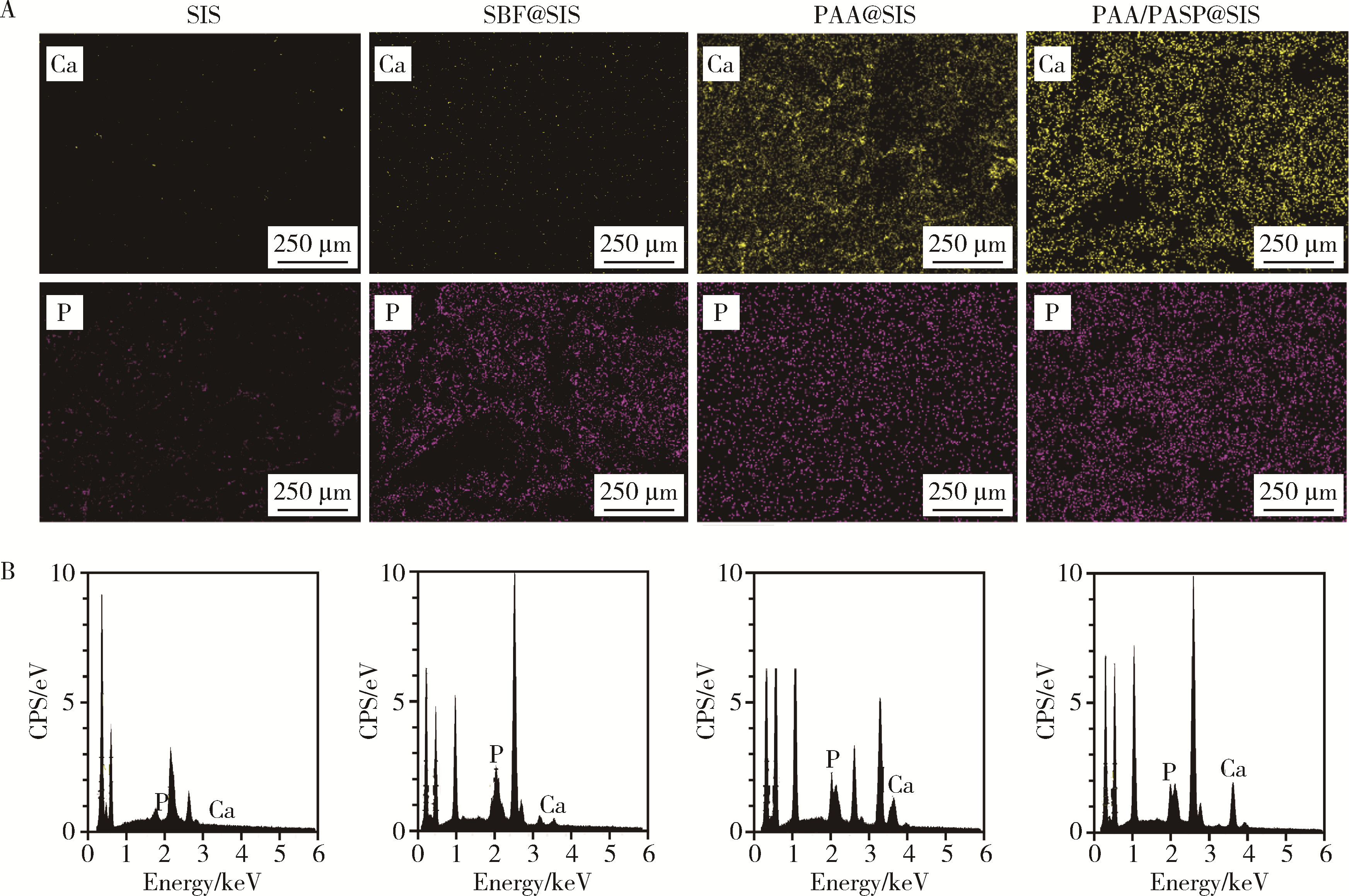
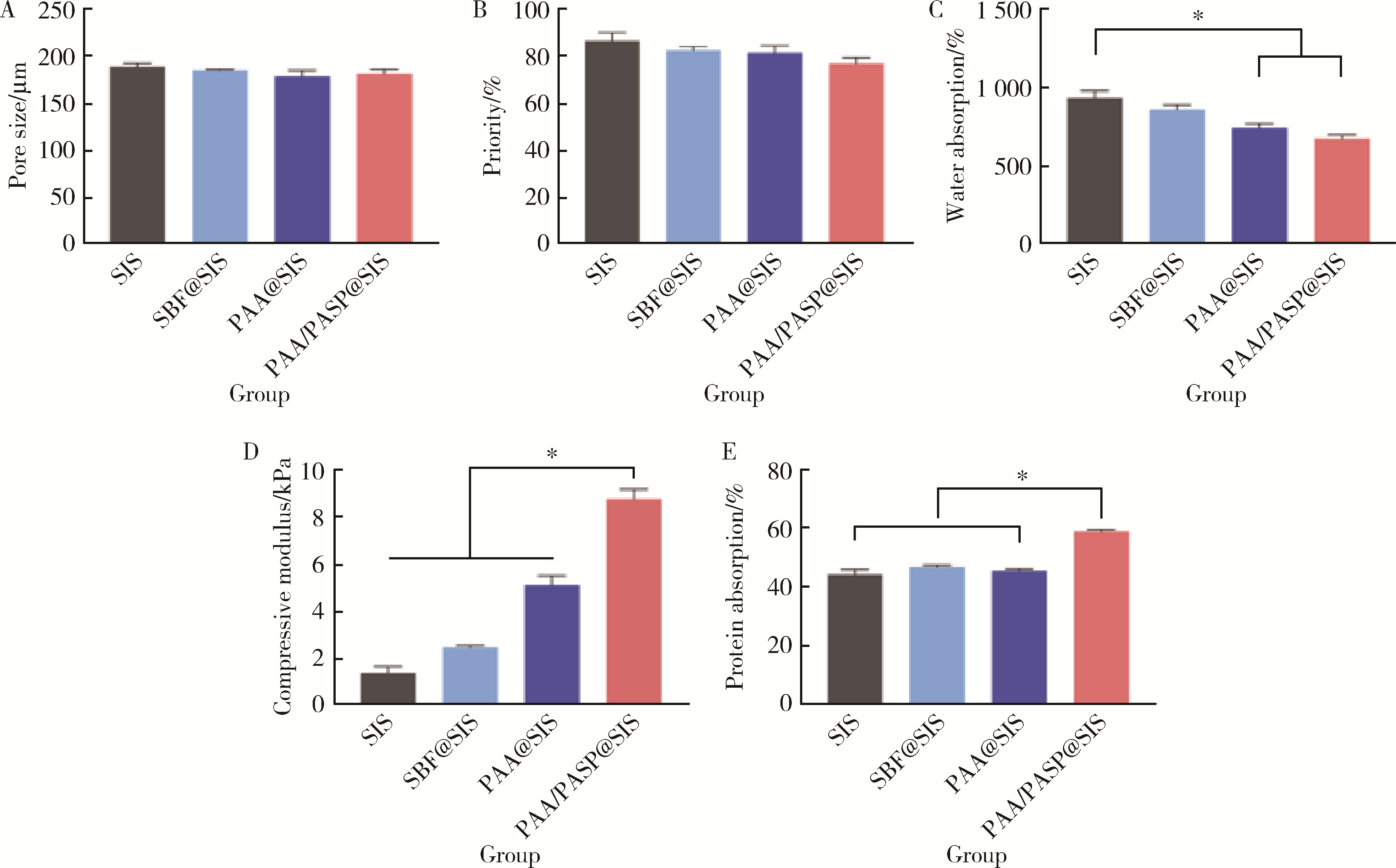
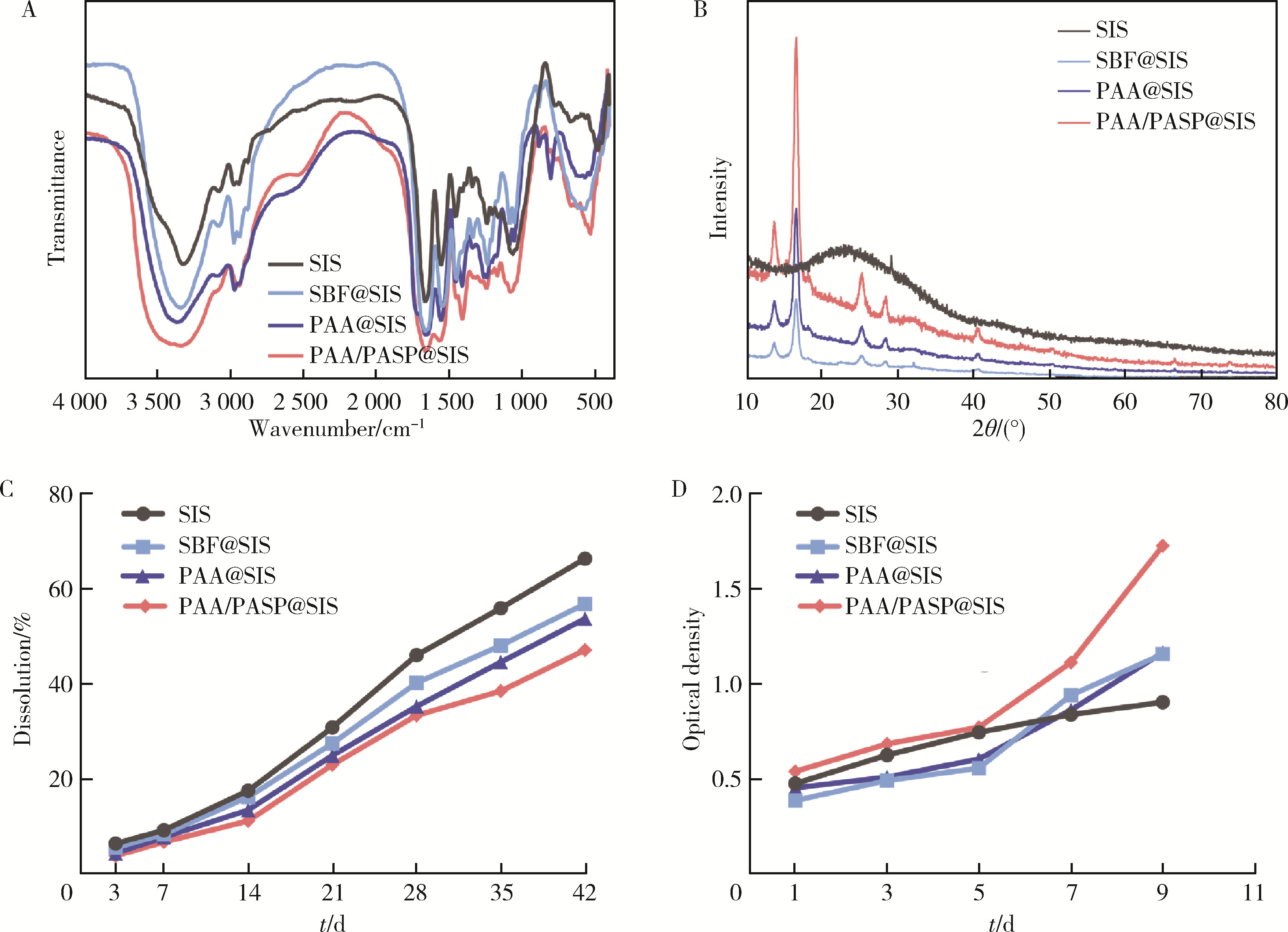
Journal of Peking University (Health Sciences) ›› 2024, Vol. 56 ›› Issue (1): 17-24. doi: 10.19723/j.issn.1671-167X.2024.01.004
Previous Articles Next Articles
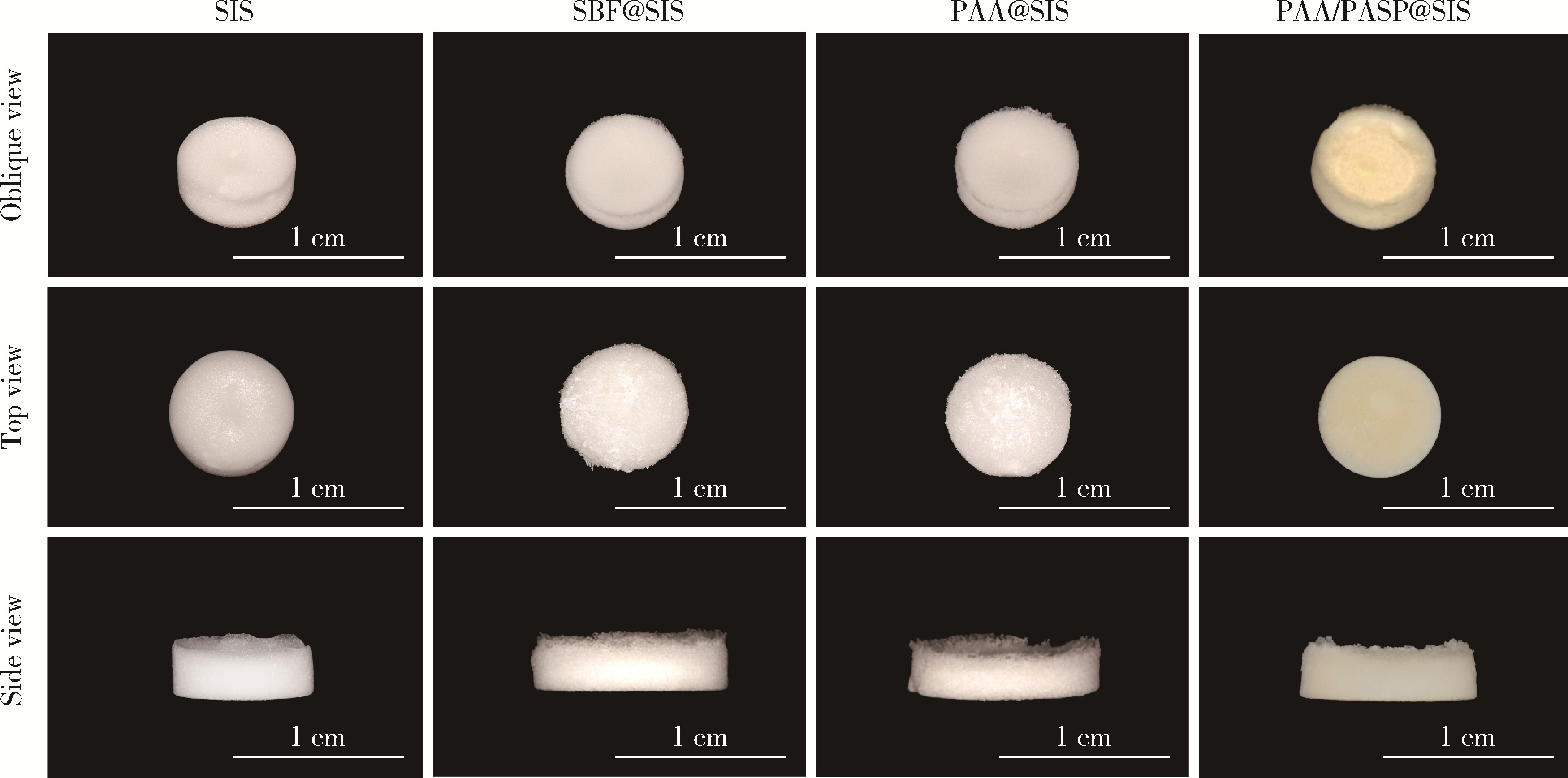
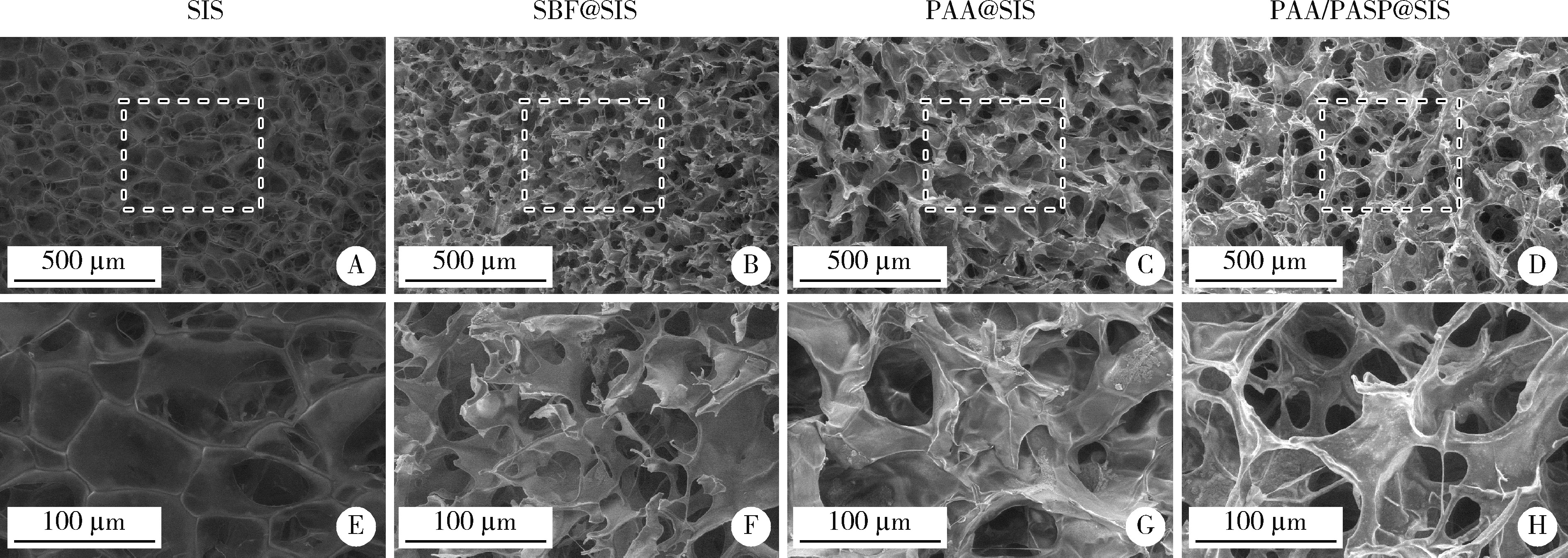
Effects of different polymers on biomimetic mineralization of small intestine submucosal scaffolds
Xiaoying CHEN1,Yi ZHANG2,Yuke LI1,Lin TANG1,*( ),Yuhua LIU1,*(
),Yuhua LIU1,*( )
)
- 1. Department of Prosthodontics, Peking University School and Hospital of Stomatology & National Center for Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Research Center of Oral Biomaterials and Digital Medical Devices & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
2. Department of General Dentistry Ⅱ, Peking University School and Hospital of Stomatology & National Center for Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Research Center of Oral Biomaterials and Digital Medical Devices & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
CLC Number:
- R318
| 1 |
Qu H , Fu H , Han Z , et al. Biomaterials for bone tissue engineering scaffolds: A review[J]. RSC Adv, 2019, 9 (45): 26252- 26262.
doi: 10.1039/C9RA05214C |
| 2 |
Langer R , Vacanti JP . Tissue engineering[J]. Science, 1993, 260 (5110): 920- 926.
doi: 10.1126/science.8493529 |
| 3 |
Asti A , Gioglio L . Natural and synthetic biodegradable polymers: Different scaffolds for cell expansion and tissue formation[J]. Int J Artif Organs, 2014, 37 (3): 187- 205.
doi: 10.5301/ijao.5000307 |
| 4 | Turnbull G , Clarke J , Picard F , et al. 3D bioactive composite scaffolds for bone tissue engineering[J]. Bioact Mater, 2018, 3 (3): 278- 314. |
| 5 | Saravanan S , Leena RS , Selvamurugan N . Chitosan based biocomposite scaffolds for bone tissue engineering[J]. Int J Biol Macromol, 2016, 93 (Pt B): 1354- 1365. |
| 6 |
Zhao J , Lu X , Duan K , et al. Improving mechanical and biological properties of macroporous HA scaffolds through composite coatings[J]. Colloids Surf B Biointerfaces, 2009, 74 (1): 159- 166.
doi: 10.1016/j.colsurfb.2009.07.012 |
| 7 |
Bian T , Zhao K , Meng Q , et al. The construction and perfor-mance of multi-level hierarchical hydroxyapatite (HA)/collagen composite implant based on biomimetic bone Haversian motif[J]. Mater Des, 2019, 162, 60- 69.
doi: 10.1016/j.matdes.2018.11.040 |
| 8 |
Ye Z , Qi Y , Zhang A , et al. Biomimetic mineralization of fibrillar collagen with strontium-doped hydroxyapatite[J]. ACS Macro Lett, 2023, 12 (3): 408- 414.
doi: 10.1021/acsmacrolett.3c00039 |
| 9 | Kato T , Suzuki T , Amamiya T , et al. Effects of macromolecules on the crystallization of CaCO3 the formation of organic/inorganic composites[J]. Supramol Sci, 1998, 5 (3): 411- 415. |
| 10 |
Aizenberg J , Addadi L , Weiner S , et al. Stabilization of amorphous calcium carbonate by specialized macromolecules in biological and synthetic precipitates[J]. Adv Mater, 1996, 8 (3): 222- 226.
doi: 10.1002/adma.19960080307 |
| 11 |
Nudelman F , Lausch A , Sommerdijk N , et al. In vitro models of collagen biominerallization[J]. J Struct Biol, 2013, 183 (2): 258- 269.
doi: 10.1016/j.jsb.2013.04.003 |
| 12 | Gower LA , Tirrell DA . Calcium carbonate films and helices grown in solutions of poly(aspartate)[J]. J Cryst Growth, 1998, 191 (1): 153- 160. |
| 13 |
Dai L , Qi Y , Niu L , et al. Inorganic-organic nanocomposite assembly using collagen as a template and sodium tripolyphosphate as a biomimetic analog of matrix phosphoprotein[J]. Cryst Growth Des, 2011, 11 (8): 3504- 3511.
doi: 10.1021/cg200663v |
| 14 | 龚春玲, 陈飞扬, 卜寿山, 等. 仿生矿化前后静电纺复合支架的性能对比[J]. 南京医科大学学报(自然科学版), 2020, 40 (5): 748- 753. |
| 15 |
Öfkeli F , Demir D , Bölgen N . Biomimetic mineralization of chitosan/gelatin cryogels and in vivo biocompatibility assessments for bone tissue engineering[J]. J Appl Polym Sci, 2021, 138 (14): e50337.
doi: 10.1002/app.50337 |
| 16 |
El-Fiqi A , Kim JK , Kim HW . Novel bone-mimetic nanohydroxyapatite/collagen porous scaffolds biomimetically minera-lized from surface silanized mesoporous nanobioglass/collagen hybrid scaffold: Physicochemical, mechanical and in vivo evaluations[J]. Mater Sci Eng C Mater Biol Appl, 2020, 110, 110660.
doi: 10.1016/j.msec.2020.110660 |
| 17 |
Li B , Wang M , Liu Y , et al. Independent effects of structural optimization and resveratrol functionalization on extracellular matrix scaffolds for bone regeneration[J]. Colloids Surf B Biointerfaces, 2022, 212, 112370.
doi: 10.1016/j.colsurfb.2022.112370 |
| 18 |
Wang M , Li B , Liu Y , et al. A novel bionic extracellular matrix polymer Scaffold enhanced by calcium silicate for bone tissue engineering[J]. ACS Omega, 2021, 6 (51): 35727- 35737.
doi: 10.1021/acsomega.1c05623 |
| 19 | Antoniac IV , Antoniac A , Vasile E , et al. In vitro characterization of novel nanostructured collagen-hydroxyapatite composite scaffolds doped with magnesium with improved biodegradation rate for hard tissue regeneration[J]. Bioact Mater, 2021, 6 (10): 3383- 3395. |
| 20 |
Saxena N , Mizels J , Cremer M , et al. Comparison of synthetic vs. biogenic polymeric process-directing agents for intrafibrillar mine-ralization of collagen[J]. Polymers (Basel), 2022, 14 (4): 775.
doi: 10.3390/polym14040775 |
| 21 |
Bharadwaz A , Jayasuriya AC . Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration[J]. Mater Sci Eng C Mater Biol Appl, 2020, 110, 110698.
doi: 10.1016/j.msec.2020.110698 |
| 22 |
Chandika P , Ko SC , Oh GW , et al. Fish collagen/alginate/chitooligosaccharides integrated scaffold for skin tissue regeneration application[J]. Int J Biol Macromol, 2015, 81, 504- 513.
doi: 10.1016/j.ijbiomac.2015.08.038 |
| 23 |
Oosterlaken BM , Vena MP , de With G . In vitro mineralization of collagen[J]. Adv Mater, 2021, 33 (16): e2004418.
doi: 10.1002/adma.202004418 |
| 24 |
Du T , Niu Y , Liu Y , et al. Physical and chemical characterization of biomineralized collagen with different microstructures[J]. J Funct Biomater, 2022, 13 (2): 57.
doi: 10.3390/jfb13020057 |
| 25 |
Yuwono LA , Siswanto , Sari M , et al. Fabrication and characte-rization of hydroxyapatite-polycaprolactone-collagen bone scaffold by electrospun nanofiber[J]. Int J Polym Mater, 2023, 72 (16): 1281- 1293.
doi: 10.1080/00914037.2022.2097675 |
| 26 | Guo M , Pegoraro AF , Mao A , et al. Cell volume change through water efflux impacts cell stiffness and stem cell fate[J]. Proc Natl Acad Sci USA, 2017, 114 (41): E8618- E8627. |
| 27 | Tang L , Chen X , Wang M , et al. A biomimetic in situ mineralization ECM composite scaffold to promote endogenous bone regeneration[J]. Colloids Surf B Biointerfaces, 2023, 232, 113587. |
| [1] | Yuan PAN,Hang GU,Han XIAO,Lijun ZHAO,Yiman TANG,Wenshu GE. Ubiquitin-specific protease 42 regulates osteogenic differentiation of human adipose-derived stem cells [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 9-16. |
| [2] | Yu-ke LI,Mei WANG,Lin TANG,Yu-hua LIU,Xiao-ying CHEN. Effect of pH on the chelation between strontium ions and decellularized small intestinal submucosal sponge scaffolds [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 44-51. |
| [3] | Yi DENG,Yi ZHANG,Bo-wen LI,Mei WANG,Lin TANG,Yu-hua LIU. Effects of different crosslinking treatments on the properties of decellularized small intestinal submucosa porous scaffolds [J]. Journal of Peking University (Health Sciences), 2022, 54(3): 557-564. |
| [4] | LAN Lin,HE Yang,AN Jin-gang,ZHANG Yi. Relationship between prognosis and different surgical treatments of zygomatic defects: A retrospective study [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 356-362. |
| [5] | HUANG Li-dong,GONG Wei-yu,DONG Yan-mei. Effects of bioactive glass on proliferation, differentiation and angiogenesis of human umbilical vein endothelial cells [J]. Journal of Peking University (Health Sciences), 2021, 53(2): 371-377. |
| [6] | Mei WANG, Bo-wen LI, Si-wen WANG, Yu-hua LIU. Preparation and osteogenic effect study of small intestinal submucosa sponge [J]. Journal of Peking University (Health Sciences), 2020, 52(5): 952-958. |
| [7] | Wei-yi WU,Bo-wen LI,Yu-hua LIU,Xin-zhi WANG. Biodegradation properties of multi-laminated small intestinal submucosa [J]. Journal of Peking University (Health Sciences), 2020, 52(3): 564-569. |
| [8] | Bo-wen LI,Wei-yi WU,Lin TANG,Yi ZHANG,Yu-hua LIU. Barrier effect of improved porcine small intestinal submucosa absorbable membrane on early healing of mandibular defects in rabbits [J]. Journal of Peking University(Health Sciences), 2019, 51(5): 887-892. |
| [9] | Chen LIANG,Wei-yu ZHANG,Hao HU,Qi WANG,Zhi-wei FANG,Ke-xin XU. Comparison of effectiveness and complications between two different methods of augmentation cystoplasty [J]. Journal of Peking University(Health Sciences), 2019, 51(2): 293-297. |
| [10] | Rong LI,Ke-long CHEN,Yong WANG,Yun-song LIU,Yong-sheng ZHOU,Yu-chun SUN. Establishment of a 3D printing system for bone tissue engineering scaffold fabrication and the evaluation of its controllability over macro and micro structure precision [J]. Journal of Peking University(Health Sciences), 2019, 51(1): 115-119. |
| [11] | WANG Zi-cheng, CHENG Li, LV Tong-de, SU Li, LIN Jian, ZHOU Li-qun. Inflammatory priming adipose derived stem cells significantly inhibit the proliferation of peripheral blood mononuclear cells [J]. Journal of Peking University(Health Sciences), 2018, 50(4): 590-594. |
| [12] | GONG Wei-yu, LIU Shao-qing, DONG Yan-mei, GAO Xue-jun, CHEN Xiao-feng. Nano-sized bioactive glass enhances osteogenesis of critical bone defect in rabbits#br# [J]. Journal of Peking University(Health Sciences), 2018, 50(1): 42-48. |
| [13] | SUI Hua-xin, LV Pei-jun, WANG Yu-guang, WANG Yong, SUN Yu-chun. Effect of lowlevel laser irradiation on proliferation and osteogenic differentiation of human adipose-derived stromal cells [J]. Journal of Peking University(Health Sciences), 2017, 49(2): 337-343. |
| [14] | . A novel tissue-engineered bone constructed by using human adipose-derived #br# stem cells and biomimetic calcium phosphate scaffold coprecipitated with #br# bone morphogenetic protein-2 [J]. Journal of Peking University(Health Sciences), 2017, 49(1): 6-015. |
|
||