Journal of Peking University (Health Sciences) ›› 2024, Vol. 56 ›› Issue (4): 673-679. doi: 10.19723/j.issn.1671-167X.2024.04.021
Previous Articles Next Articles
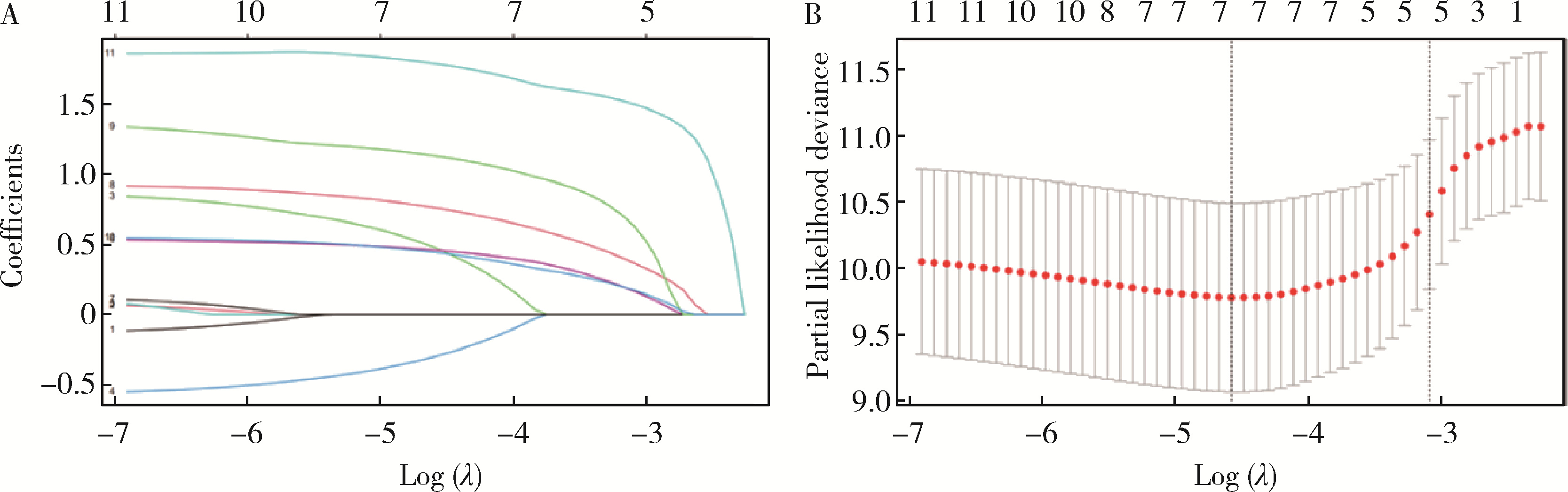
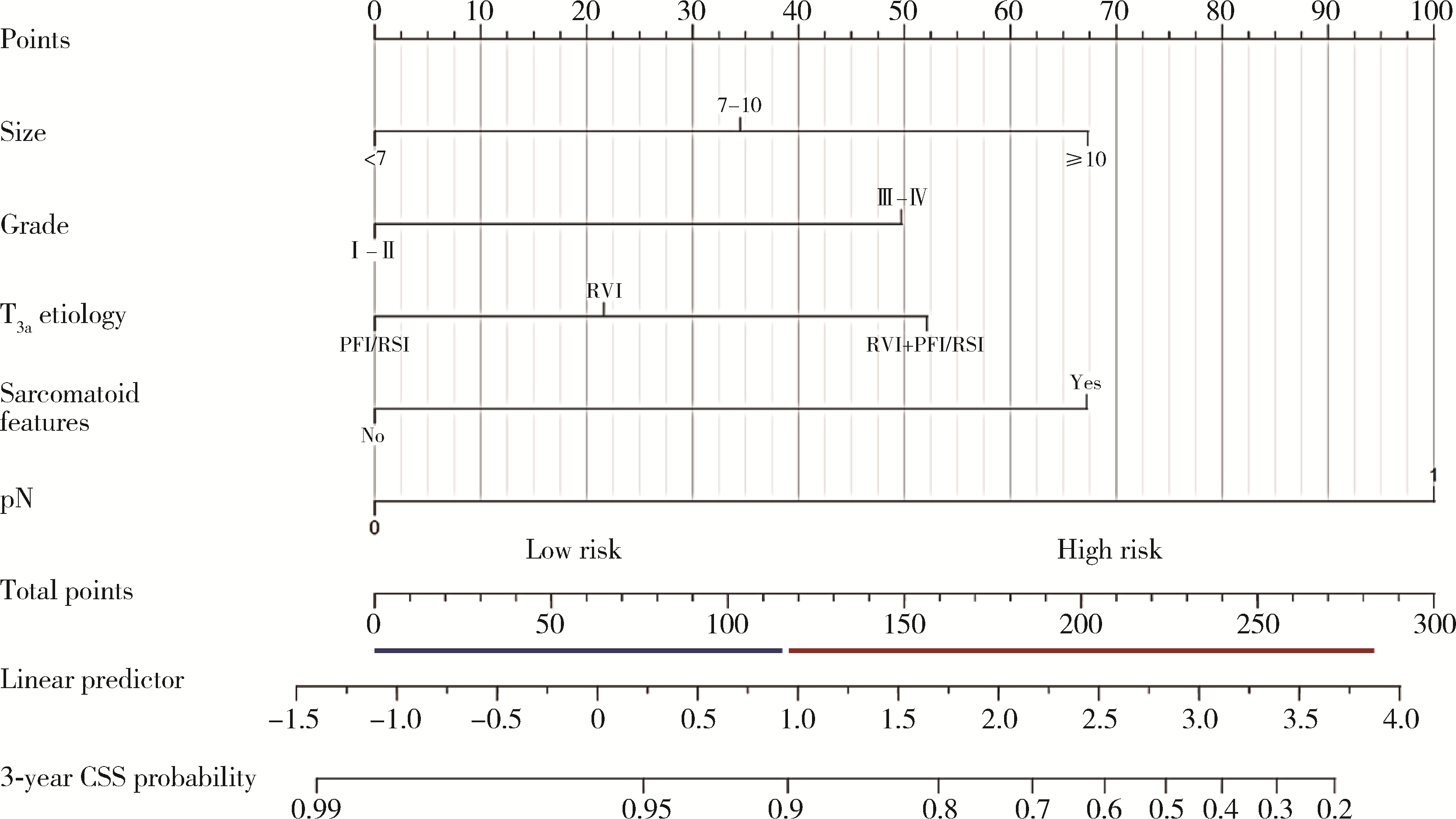
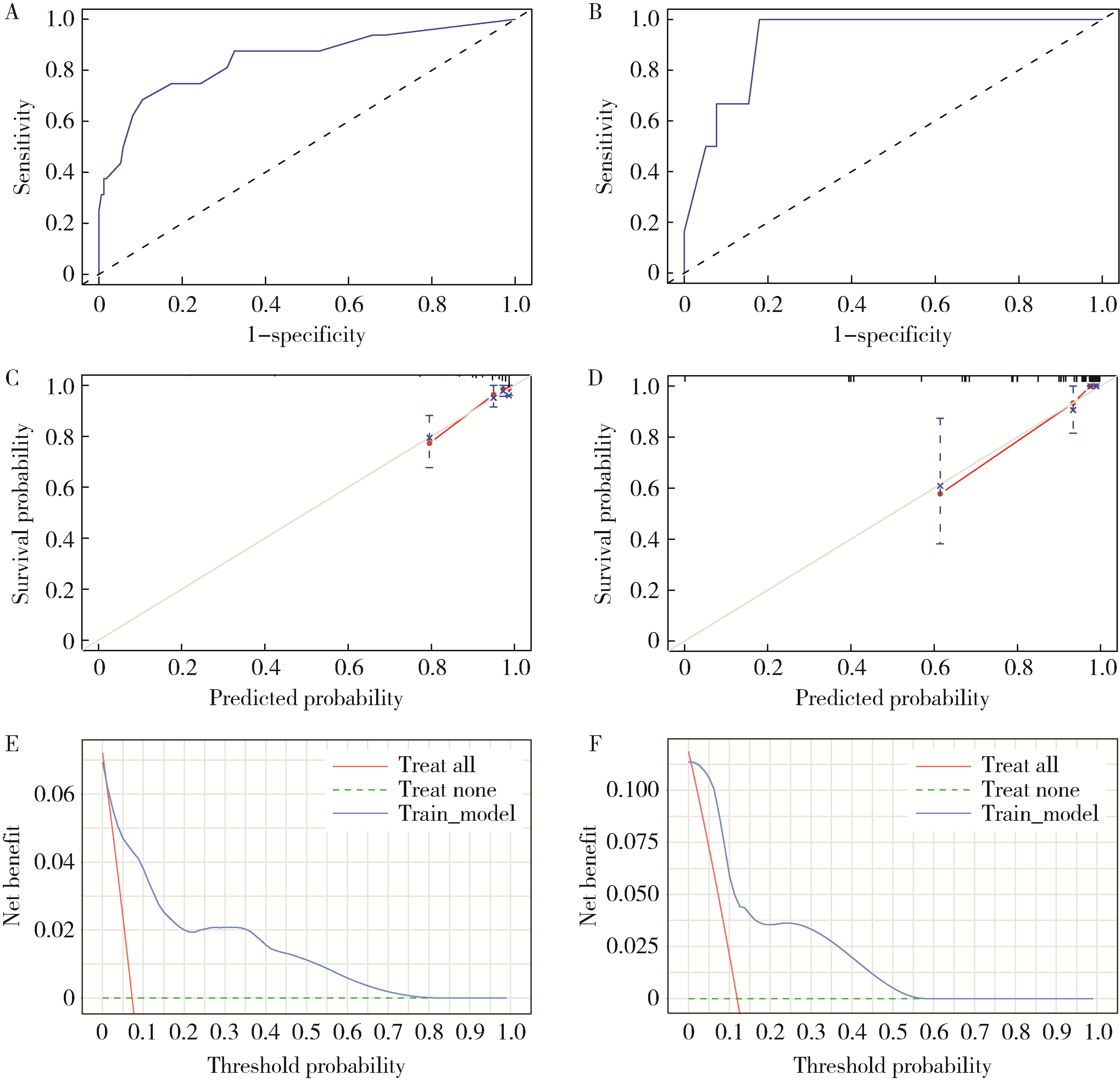
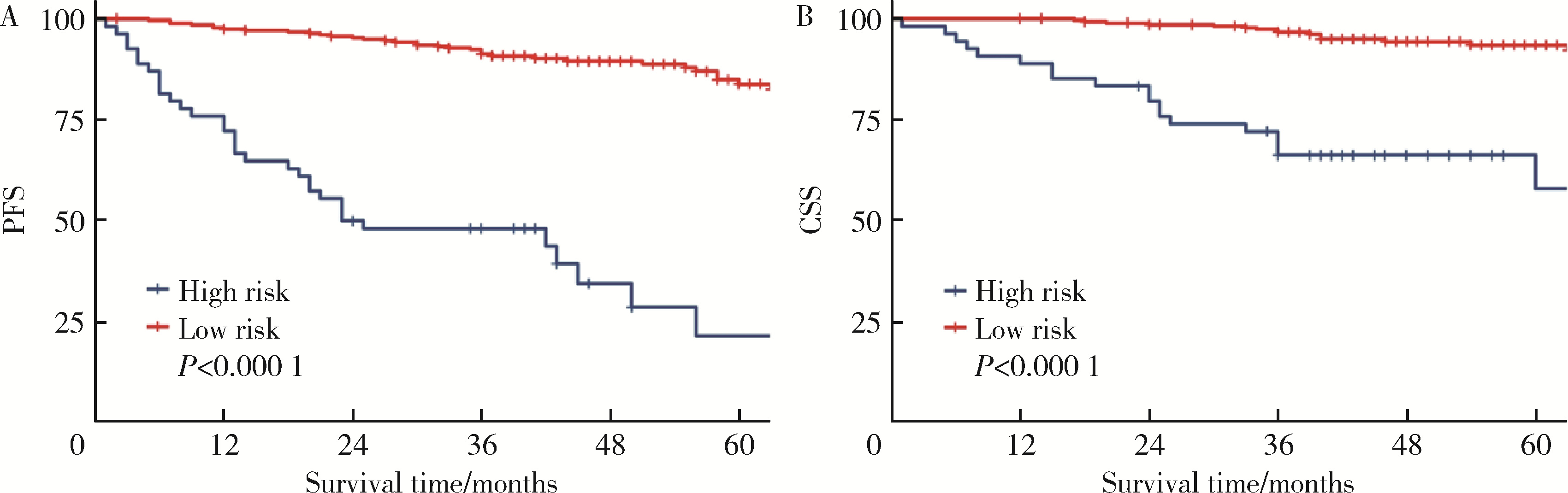
Predicting the 3-year tumor-specific survival in patients with T3a non-metastatic renal cell carcinoma
Zezhen ZHOU,Shaohui DENG,Ye YAN,Fan ZHANG,Yichang HAO,Liyuan GE,Hongxian ZHANG,Guoliang WANG,Shudong ZHANG*( )
)
- Department of Urology, Peking University Third Hospital, Beijing 100191, China
CLC Number:
- R737.11
| 1 |
Elkassem AA , Allen BC , Sharbidre KG , et al. Update on the role of imaging in clinical staging and restaging of renal cell carcinoma based on the AJCC 8th edition, from the AJR special series on cancer staging[J]. AJR Am J Roentgenol, 2021, 217 (3): 541- 555.
doi: 10.2214/AJR.21.25493 |
| 2 |
Guo P , Wang Y , Han Y , et al. Development and validation of a nomogram to predict postoperative cancer-specific survival of patients with nonmetastatic T3a renal cell carcinoma[J]. Urol Oncol, 2021, 39 (12): 835.e19- 835.e27.
doi: 10.1016/j.urolonc.2021.06.014 |
| 3 |
Musso G , Fallara G , Rosiello G , et al. Differential prognostic value of extrarenal involvement in patients with non-metastatic renal cell cancer[J]. Clin Genitourin Cancer, 2023, 21 (4): e279- e285.e1.
doi: 10.1016/j.clgc.2023.02.008 |
| 4 |
Shah PH , Lyon TD , Lohse CM , et al. Prognostic evaluation of perinephric fat, renal sinus fat, and renal vein invasion for patients with pathological stage T3a clear-cell renal cell carcinoma[J]. BJU Int, 2019, 123 (2): 270- 276.
doi: 10.1111/bju.14523 |
| 5 |
Li L , Shi L , Zhang J , et al. The critical impact of tumor size in predicting cancer special survival for T3aM0M0 renal cell carcinoma: A proposal of an alternative T3aN0M0 stage[J]. Cancer Med, 2021, 10 (2): 605- 614.
doi: 10.1002/cam4.3629 |
| 6 |
Lam JS , Klatte T , Patard JJ , et al. Prognostic relevance of tumour size in T3a renal cell carcinoma: A multicentre experience[J]. Eur Urol, 2007, 52 (1): 155- 162.
doi: 10.1016/j.eururo.2007.01.106 |
| 7 |
Tan WS , Koelker M , Campain N , et al. Comparison of long-term outcomes for young and healthy patients with cT1a and cT3a renal cell carcinoma treated with partial nephrectomy[J]. Eur Urol Focus, 2023, 9 (2): 333- 335.
doi: 10.1016/j.euf.2022.09.018 |
| 8 | Chung DY , Kang DH , Kim JW , et al. Comparison of oncologic outcomes between partial nephrectomy and radical nephrectomy in patients who were upstaged from cT1 renal tumor to pT3a renal cell carcinoma: An updated systematic review and meta-analysis[J]. Ther Adv Urol, 2020, 12, 1756287220981508. |
| 9 |
Deng H , Fan Y , Yuan F , et al. Partial nephrectomy provides equivalent oncologic outcomes and better renal function preservation than radical nephrectomy for pathological T3a renal cell carcinoma: A meta-analysis[J]. Int Braz J Urol, 2021, 47 (1): 46- 60.
doi: 10.1590/s1677-5538.ibju.2020.0167 |
| 10 |
Liu H , Kong QF , Li J , et al. A meta-analysis for comparison of partial nephrectomy vs. radical nephrectomy in patients with pT3a renal cell carcinoma[J]. Transl Androl Urol, 2021, 10 (3): 1170- 1178.
doi: 10.21037/tau-20-1262 |
| 11 |
Liu Z , Yang Z , Li J , et al. Partial versus radical nephrectomy for the treatment of pT3aN0M0 renal cell carcinoma: A propensity score analysis[J]. Asian J Surg, 2023, 46 (9): 3607- 3613.
doi: 10.1016/j.asjsur.2023.04.058 |
| [1] | Yuanmei LIU, Yicheng FU, Jingxin HAO, Fuchun ZHANG, Huilin LIU. Construction and validation of a nomogram for predicting in-hospital postoperative heart failure in elderly patients with hip fracture [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 874-883. |
| [2] | Zhicun LI, Tianyu WU, Lei LIANG, Yu FAN, Yisen MENG, Qian ZHANG. Risk factors analysis and nomogram model construction of postoperative pathological upgrade of prostate cancer patients with single core positive biopsy [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 896-901. |
| [3] | Junyong OU,Kunming NI,Lulin MA,Guoliang WANG,Ye YAN,Bin YANG,Gengwu LI,Haodong SONG,Min LU,Jianfei YE,Shudong ZHANG. Prognostic factors of patients with muscle invasive bladder cancer with intermediate-to-high risk prostate cancer [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 582-588. |
| [4] | Shuai LIU,Lei LIU,Zhuo LIU,Fan ZHANG,Lulin MA,Xiaojun TIAN,Xiaofei HOU,Guoliang WANG,Lei ZHAO,Shudong ZHANG. Clinical treatment and prognosis of adrenocortical carcinoma with venous tumor thrombus [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 624-630. |
| [5] | Le YU,Shaohui DENG,Fan ZHANG,Ye YAN,Jianfei YE,Shudong ZHANG. Clinicopathological characteristics and prognosis of multilocular cystic renal neoplasm of low malignant potential [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 661-666. |
| [6] | Fan SHU,Yichang HAO,Zhanyi ZHANG,Shaohui DENG,Hongxian ZHANG,Lei LIU,Guoliang WANG,Xiaojun TIAN,Lei ZHAO,Lulin MA,Shudong ZHANG. Functional and oncologic outcomes of partial nephrectomy for cystic renal cell carcinoma: A single-center retrospective study [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 667-672. |
| [7] | Yangyi FANG,Qiang LI,Zhigao HUANG,Min LU,Kai HONG,Shudong ZHANG. Well-differentiated papillary mesothelial tumour of the tunica vaginalis: A case report [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 741-744. |
| [8] | Yuanyuan ZENG,Yun XIE,Daonan CHEN,Ruilan WANG. Related factors of euthyroid sick syndrome in patients with sepsis [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 526-532. |
| [9] | Jian-bin LI,Meng-na LYU,Qiang CHI,Yi-lin PENG,Peng-cheng LIU,Rui WU. Early prediction of severe COVID-19 in patients with Sjögren’s syndrome [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1007-1012. |
| [10] | Yun-chong LIU,Zong-long WU,Li-yuan GE,Tan DU,Ya-qian WU,Yi-meng SONG,Cheng LIU,Lu-lin MA. Mechanism of nuclear protein 1 in the resistance to axitinib in clear cell renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 781-792. |
| [11] | Huan-rui LIU,Xiang PENG,Sen-lin LI,Xin GOU. Risk modeling based on HER-2 related genes for bladder cancer survival prognosis assessment [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 793-801. |
| [12] | Zi-xuan XUE,Shi-ying TANG,Min QIU,Cheng LIU,Xiao-jun TIAN,Min LU,Jing-han DONG,Lu-lin MA,Shu-dong ZHANG. Clinicopathologic features and prognosis of young renal tumors with tumor thrombus [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 802-811. |
| [13] | Dong LAN,Zhuo LIU,Yu-xuan LI,Guo-liang WANG,Xiao-jun TIAN,Lu-lin MA,Shu-dong ZHANG,Hong-xian ZHANG. Risk factors for massive hemorrhage after radical nephrectomy and removal of venous tumor thrombus [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 825-832. |
| [14] | Han LU,Jian-yun ZHANG,Rong YANG,Le XU,Qing-xiang LI,Yu-xing GUO,Chuan-bin GUO. Clinical factors affecting the prognosis of lower gingival squamous cell carcinoma [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 702-707. |
| [15] | Yun-fei SHI,Hao-jie WANG,Wei-ping LIU,Lan MI,Meng-ping LONG,Yan-fei LIU,Yu-mei LAI,Li-xin ZHOU,Xin-ting DIAO,Xiang-hong LI. Analysis of clinicopathological and molecular abnormalities of angioimmunoblastic T-cell lymphoma [J]. Journal of Peking University (Health Sciences), 2023, 55(3): 521-529. |
|
||